- Volume 62 , Number 4
- Page: 521–6
Leprosy and infection with the human immunodeficiency virus in Uganda; a case-control study
ABSTRACT
Both leprosy and infection with the human immunodeficiency virus (HIV) are endemic in Uganda. Various speculations about a possible interaction between the two infections have been put forward but not confirmed. A case-control study involving 189 new leprosy patients and 481 matched controls, resident in eight Ugandan districts, was carried out to investigate if any relationship exists between leprosy and infection with HIV-1 in Uganda. Scrum samples f rom 23 (12.2%) of the 189 leprosy patients tested positive for HIV-1 antibodies as compared to 88 (18.3%) of the 481 control sera. The two proportions of HIV seropositivity are not different statistically. A stratified analysis of the data by districts was done and showed a negative relationship between leprosy and HIV infection in the case of Rakai District (0.04 < odds ratio < 0.61, p = 0.002). It is recommended that studies seeking to observe the clinical progress of dually infected patients might help to reveal new knowledge about a possible relationship between HIV and leprosy and about the immunology of leprosy in general.RÉSUMÉ
La lèpre et l'infection par le virus de l'immunodéficience humaine (VIH) sont toutes deux endémiques en Ouganda. Diverses hypothèses quant à une interaction possible entre les deux infections ont été avancées, mais non confirmées.Une étude cas-témoins comprenant 189 nouveaux malades de la lèpre et 481 témoins appariés, résidant dans 8 Districts d'Ouganda, a été réalisée pour rechercher s'il existait une relation entre la lèpre et l'infection à VIH en Ouganda. Des échantillons de serum de 23 ( 12.2%) des 189 malades de la lèpre ont été testés positifs pour les anticorps vis-à-vis du VIH-1, comparés à 88 (18.3%) des scrums des 481 témoins. Les deux proportions de séropositivité vis-à-vis du VIH ne sont pas significativement différentes. Une analyse des données par district a été réalisée et a montré une relation négative entre la lèpre et l'infection VIH dans le cas du District de Rakai (0.04 < odds ratio < 0.61, p = 0.002).
Des études ayant pour objectif l'observation de la progression clinique des malades doublement infectés pourraient aider à une avancée des connaissances quant aux relations possibles entre le VIH et la lèpre et quant à l'immunologie de la lèpre en général.
RESUMEN
En Uganda, la lepra y la infección por el virus de la inmunodeficiencia humana son endémicas. Aunque existe la sospecha de interrelación entre las dos enfermedades, no hay estudios suficientes que confirmen lo anterior. Por esta razón, para investigar si existe alguna relación entre la lepra y la infección por VIH-1, se efectuó un estudio de casos controlados en 189 pacientes con lepra de diagnóstico reciente y en 481 controles "aparcados," habitantes de 8 distritos ugandeses. Veintitrés muestras de suero (12.2%) de los 189 pacientes con lepra y 88 (18.3%) de los 481 sueros control, resultaron positivos para VIH-1; la proporción de casos positivos en cada grupo no difirió de manera significativa. El análisis estratificado de los datos en los diferentes distritos, mostró una relación negativa entre lepra y la infección por VHI en el Distrito de Rakai (0.04-0.61, p = 0.002).Los estudios diseñados para observar el progreso clínico de los pacientes con las dos infecciones, podrían ayudar a entender las posibles intcrrclaciones entre VHI y lepra, y la inmunología de la lepra en general.
The acquired immunodeficiency syndrome (AIDS) and leprosy are both endemic in Uganda. As of 1 November 1992, the cumulative number of AIDS cases in Uganda was reported as 34,611 (17). Uganda also was included among 25 African countries with a significant leprosy problem (10). At the end of 1992, the prevalence of registered leprosy patients in the country was reported as 1.3 per 10,000 population with a new case detection rate of about 1.1 per 10,000 population (Uganda National Tuberculosis/Leprosy Programme. Annual Leprosy Statistics, 1992).
Various speculations about the possible interaction between infection with the human immunodeficiency virus (HIV) and leprosy have been recorded (6,9,11,16). It has been suggested that the effect of HI V on cellmediated immunity can lead to conversion of subclinical cases to tuberculoid cases, downgrading of paucibacillary (PB) cases to multibacillary (MB) cases, and an increased incidence of type 2 leprosy reactions (16).
This case-control study was carried out to investigate if there is any relationship between HIV scropositivity and leprosy in Uganda.
MATERIALS AND METHODS
The study was carried out in part of the leprosy control project area of the St. Francis Leprosy Centre, Buluba. The area, covering eight Ugandan districts, has a population of about 4,910,700 persons (Republic of Uganda Provisional Results of the 1991 Population-Housing Census). Leprosy case detection in the area occurs mainly through voluntary reporting. The patients, once diagnosed, are looked after through a network of clinics which are professionally supervised from St. Francis Leprosy Centre, Buluba.
All newly detected cases of leprosy from among the resident population of the study area during January 1991 to June 1992 were requested to participate in the study but were only included in the study if they consented. All cases registered in this study reported voluntarily for diagnosis. The cases were given a clinical examination following a standard Ugandan Ministry of Health format. Skin smears were taken in and reported on at the laboratory of St. Francis Leprosy Centre, Buluba.
Using the clinical data and results of the skin-smear examinations, the patients were classified as tuberculoid (TT), borderline tuberculoid (BT), borderline lepromatous (BL) and lepromatous (LL) as per the Ridley-Jopling scale (13). The diagnoses and classifications initially made by paramedical supervisors were confirmed by medical officers. Histopathological examinations were only carried out in doubtful cases.
Up to three controls were chosen for each patient. The controls were matched with patients for sub-county of residence and age, within 5-year age groups up to age 40, and within 10-year age groups thereafter. Matching by sub-county of residence was achieved by selecting suitable controls from the nearest households. Only one adult female from each household was recruited into each matched set. In the case of those few leprosy patients below the age of 15 years, an attempt was made to select controls within a 2-year age difference. There is a high chance that young patients would be HIV negative and that controls up to 5 years older may have a higher HIV seroprevalence.
Cases and controls were visited at their homes by paramedical supervisors. Information obtained from the visits was recorded on the questionnaires designed for that purpose. Five ml blood samples were collected from each subject and transported on wet ice to one of two laboratories: the Tuberculosis Reference Laboratory in Kampala and the laboratory of St. Francis Leprosy Centre, Buluba. Scrum was obtained from the samples by centrifugation. The laboratories were provided with forms bearing details of study number, age and sex of the donor subjects but not indicating their names or whether they were leprosy patients or controls.
The sera were then stored in refrigerators at 4ºC-8ºC and as soon as possible aliquots were screened for HIV antibodies with HIV-Check Dupont Kits. The sera were subsequently tested again by competitive ELISA (Wellcozyme and Behring Enzygnost anti-HIV); the latter tests were carried out by the Uganda Virus Research Institute (UVRI), Entebbe. Sera found positive by a consensus of the ELISA results were considered positive; any results found discrepant were confirmed by Western blotting.
The data were entered into a computer and analyzed using EPI INFO (version 5) software (June 1990 edition). Frequencies of demographic factors, i.e., sex, age and district of origin, were computed as well as the proportions of HIV seropositives for both case and control groups. The overall association by district was calculated using the x2 test and Fisher's exact test. A Mantel-Haenszel analysis for the effects of sex and leprosy type also was done.
RESULTS
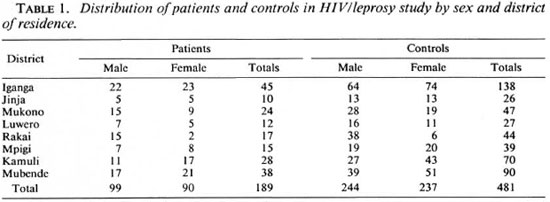
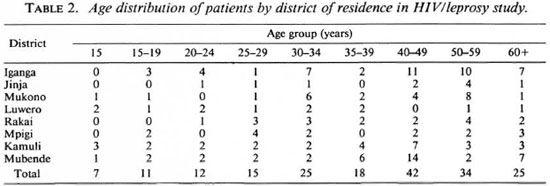
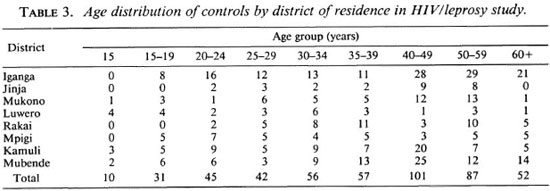
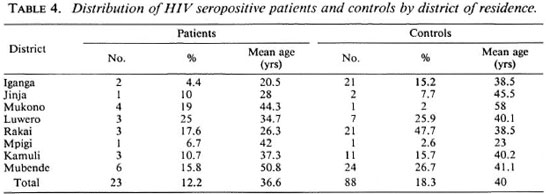
Altogether 189 leprosy patients and 481 matched controls were recruited into the study. Of the 189 patients 99 (52.4%) were male and 90 (47.6%) were female; while 244 (50.7%) of the 481 controls were male and 237 (49.3%) were female. The mean age of the patients was 40.5 years; that of the controls was 39.5 years (the difference between the two is not statistically significant). The distribution of the cases and controls by sex, district of residence, and age is summarized in Tables 1 through 3. Twenty-three (12.2%) of the serum samples from the 189 leprosy patients tested positive for HIV antibodies. Out of 481 serum samples collected from controls, 88 (18.3%) were positive for HIV antibodies. The distribution of HIV-seropositivc patients and controls by district of residence is shown in Table 4.
Tests of association for the whole sample population had a p value of p = 0.176. Going by this result, we conclude that there is not enough evidence to show that HIV seropositivity and leprosy arc related. When a Cochran-Mantel-Haenszel analysis was used to test the hypothesis of no association using the districts as a controlling factor, the results were very similar, p = 0.198.
When the test was repeated for each of the individual districts and the results of Fisher's exact test for association analyzed, in all the districts except Rakai, no association was found. A reversed association was found in the case of Rakai District (0.04 < odds ratio (OR) < 0.61, p = 0.00175).
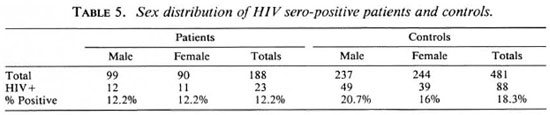
The proportion of HIV-seropositive leprosy patients was almost equal in males and females. In the control group there were more HIV-seropositive males than females (Table 5).
Of the 189 leprosy patients studied, 138 (73%) had PB leprosy and the remaining 51 (27%) had MB leprosy. Out of the 23 HIVseropositive patients, 12 (52.2%) were PB and 11 (47.8%) were MB. In this population of leprosy patients HIV seropositivity was significantly more frequent among MB than PB patients (Table 6).

DISCUSSION
It has been recorded that the selective tropism of HIV-1 for T-4 lymphocytes leads to T-ccll depletion and the lowering of T-cell defenses, giving way to a variety of opportunistic infections (4). Several studies have already shown that there exists a number of leprosy patients who are dually infected with HIV-1 and Mycobacterium leprae (7,8). Wc report here 23 patients (perhaps the largest number reported from a single study) arising from a population where both AIDS and leprosy are significant public health problems. The outcome of this case-control study, although not necessarily representing the situation in the whole of Uganda, concurs with the conclusions drawn by others (11,16) that there is, so far, not enough evidence to show that leprosy and HIV scropositivity are related.
The results of this study show that infection with HIV-1 may be expected to occur in about 12% of newly recognized leprosy patients in Uganda. As long as both HIV-1 infection and leprosy continue to be endemic in Uganda, it remains important to study the implications of the dual infection on the clinical presentation of leprosy, the response to chemotherapy, the frequency and pattern of leprosy reactions, the outcome of their management, and the influence on relapse rates. Some of the observations made on the dually infected patients in the course of this study have been published (2) elsewhere.
A pattern similar to the one in Rakai District where HIV-1 infection was significantly more frequent in the control population than among the new leprosy patients, has been recorded in at least one other study (14). Although this could be simply the results of a sampling error or other methodological biases, we find it hard to ignore the observation, since Rakai District has one of the most severe HIV-1/AIDS problems in Uganda (Uganda Ministry of Health AIDS Surveillance Report, December 1991).
It has been suggested that the immunological changes in lenti-virus infections may resemble those of lepromatous leprosy (1). In the population studied, HIV-1 scropositivity was significantly more frequent among MB than PB patients. The mean age of the HIV-1 seropositive patients did not differ significantly (39.2 years in PB and 37.9 years in MB).
Earlier observations on ELISA and Western blot (WB) assays used for confirmation of HIV-1 infection had indicated that falsepositivity and crossreactivity by WB assays could occur (3,12). A particularly high rate of crossreactivity, 64%, was reported from India by Shiraj and others (15). These authors recommended that in countries where leprosy and tuberculosis arc highly prevalent, the results of the WB assays should be interpreted with great caution. They suspected that there could be a common antigenic pattern between HIV-1 and mycobacteria, especially M. leprae. In our study, WB was only employed in cases where results from ELISAs were found discrepant. Some further insight into this situation may be gained through continuing to follow up the seropositive patients in this study with the view to observing the transformation from HIV infection to AIDS.
The inter-rclationship between HIV-1 infection and leprosy still deserves further study because so far the immunology of leprosy has not yet been exhaustively studied and the absence of a relationship between the two remains hard to explain.
Acknowledgment. We thank, G. Emukeu, M. Kawikizi, A. Mugerwa, M. Mugenyi, S. Musoke, D. Mwandha, M. Kawuma, C. Gateera, E. Hantangimana, B. Akurut and F. Ekitui, all of whom contributed in different ways to the collection and recording of the data in this study. Thanks also arc due to the Director of the Uganda Virus Research Institute (UVRI), Entebbe, for facilitating confirmatory testing of our serum samples in the Institute. The UVRI/MRC Project Staff assisted us in the data analysis. We arc grateful to the Director of Medical Services of the Uganda Ministry of Health for permission to publish this paper.
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR).
REFERENCES
1. BARNASS , S. Lcntiviruses and mycobacterial disease. Immunol. Today 8(1987)9.
2. BWIRE, R. and KAWUMA, H. J. S. Type 1 reaction, neuritis and steroid therapy: the impact of infection with the human immunodeficiency virus. Trans. R. Soc. Trop. Med. Hyg. 88(1994)315-316.
3. OOUROUCE, A. M., MULLER, J.-Y. and RICHARD, D. False-positive Western blot reactions to human immunodeficiency virus in blood donors. Lancet 2(1986)921-922.
4. FAUCI , A. S. Acquired immunodeficiency syndrome: epidemiologic, immunologic and therapeutic considerations. Ann. Intern. Med. 110(1984) 92-106.
5. FINE , P. E. M . Reflections on the elimination of leprosy. (Editorial) Int. J. Lepr. 60(1992)71-80.
6. FLEMING , A. F . Opportunistic infection in AIDS in developed and developing countries. Trans. R. Soc. Trop. Med. Hyg. 84 Suppl. (1990) 1-6.
7. LAMFERS, E. J. P., BASTIAANS, A. H., MRAVUNAC, M. and RAMPEN, P. H. J. Leprosy in the acquired immunodeficiency syndrome. (Letter) Ann. Intern. Med. 107(1987)111-112.
8. MEERAN , K. Prevalence of HIV infection among patients with leprosy and tuberculosis in rural Zambia. Br. Med. J. 298(1989)364-365.
9. MILLER, R. A. Leprosy and AIDS; a review of the lileratureand speculations on the impact of CD4 + lymphocyte depletion on immunity to Mycobacterium leprae. Int. J. Lepr. 59(1991)639-644.
10. NOORDEEN, K., LOPEZ BRAVO, L. and SUNDARE-SAN , T. K. Estimated number of leprosy cases in the world. Lepr. Rev. 63(1992)282-287.
11. PONNIGHAUS, J. M., MWANJASI, L. J., FLNE, P. E. M., SHAW, M.-A., TURNER, A. G, OXIIORROW, S. M., LUCAS, S. B., JENKINS, P. A., STERNE, J. A. C. and BLISS, L. IS HIV infection a risk factor for leprosy? Int. J. Lepr. 59(1991)221-228.
12. RANKI, A., JOHANSSON, E. and KROHN, K. Interpretation of antibodies reacting solely with human retroviral core proteins. N. Engl. J. Med. 318(1988) 488-489.
13. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
14. SAHA, K., CHATTOPADHYA, D., DASH, K., SAHA, U., TYAGI, P. K., GUPTA, M. M., PARAASHARI, A. and SIIARMA , A. K. Sexually transmitted diseases in leprosy patients in north and northeastern India; a futile search for human immunodeficiency virus antibody. Int. J. Lepr. 58(1990)660-665.
15. SHIVRAJ, L., PATIL, S. A., GIRDHAR, A., SENGUPTA, U., DESIKAN, K. V. and SRINIVASAN, H. Antibodies to HIV-1 in sera from patients with mycobacterial infections. Int. J. Lepr. 56(1988)546-551.
16. TURK, J. L. and REES, R.J. W. AIDS and leprosy. Lepr. Rev. 59(1988)193-194.
17. WORLD HEALTH ORGANIZATION. Weekly Epidemiol. Rec. 68(1993)9-10.
1. M.B.Ch.B., D.P.H., Dip.Derm., Medical Superintendent;
2. M.B.Ch.B., Medical Officer, St. Francis Leprosy Centre Buluba, P.O. Box 1059, Jinga, Uganda.
3. M.B.Ch.B., M.Sc, D.T.C.E., Programme Manager, National Tuberculosis/Leprosy Programme, Ministry of Health, Uganda.
Received for publication on 27 May 1993.
Accepted for publication in revised form on 10 August 1994.