- Volume 62 , Number 4
- Page: 532–8
Protective effect of BCG against leprosy and its subtypes: a case-control study in southern vietnam
ABSTRACT
A case-control study was conducted to assess the protective effect of intradermal BCG against leprosy and its subtypes in southern Vietnam. A total of 177 cases were selected with a distribution by subtypes as follows: 38 TT, 23 BT, 51 BB, 36 BL, 22 LL, and 7 indeterminate. Two controls were matched with a case for age, sex, ethnic group, socioeconomic status, and district area. The odds ratio assessing the protective effect of BCG varied f rom 0.44 (0.19-1.03) in the BB subtype to 3.00 (0.24-37.5) in indeterminate leprosy; whereas its overall value was 0.71 (0.45-1.10) for leprosy per se. When all borderline leprosy types were pooled, the protective effect of BCG was found significant with an odds ratio of 0.48 (0.27-0.84). In the polar forms of leprosy, TT and LL, the odds ratio was > 1 with large confidence intervals. It is possible that BCG induces a shift in the immune response to a higher level of cell-mediated immunity. When BCG vaccination is given after primary infection with Mycobacterium leprae, this shift could be the cause of an increase in the risk of the occurrence of milder and transient forms of the disease. In TT forms BCG might reinforce the preexisting subclinical immunopathological reactions, and in stable LL forms BCG might be unable to induce any protective form of immunity. These results confirm the important variability in the protection offered by BCG with respect to the different types of leprosy, and may have important implications for the design and the interpretation of vaccine trials that should take into account the respective proportions of leprosy forms observed in the study region.RÉSUMÉ
Une étude cas-témoins a été réalisée afín d'évaleur l'effet protecteur du BCG intradermique vis-à-vis de la lèpre et de ses sous-types dans le Sud du Vietnam. Un total de 17 cas a été sélectionné, avec la distribution par sous-types suivante: 38 TT , 23 BT, 51 BB , 36 BL, 22 LL , et 7 indéterminés. Deux témoins ont été appariés pour chaque cas pour l'âge, le sexe, le groupe ethnique, le statut socio-économique et le district. L'odds ratio évaluant l'effet protecteur du BCG variait de 0.44 (0.19-1.03) pour le sous-type BB à 3.00 (0.24 - 37.5) pour la lèpre indéterminée; alors que sa valeur globale était de 0.71 (0.45-1.10) pour la lèpre comme telle. Quand tous les types de lèpre borderline furent mis ensemble, l'effet protecteur du BCG fut trouvé significatif avec un odds ratio de 0.48 (0.27-0.84). Pour les formes polaires de la lèpre, TT et LL , l'odds ratio était supérieur à I avec de larges limites de confiance. Il est possible que le BCG induise un glissement dans la réponse immunitaire vers un taux plus élevé d'immunité à médiation cellulaire. Quand le vaccin BCG est administré après une infection primaire par le Mycobacterium leprae, ce glissement pourrait être la cause d'une augmentation du risqe de développer des formes plus bénignes et transitoires de la maladie. Dans les formes TT , le BCG pourrait renforcer les réactions immunopathologiques subcliniques pré-existantes, et dans les formes LL stables, le BCG pourrait être incapable de produire une quelconque forme d'immunité protectrice. Ces résultats confirment la variabilité importante de protection offerte par le BCG par rapport aux diflérents types de lèpre, et peuvent avoir des implications importantes pour la conception et l'interprétation des essais de vaccination qui devraient prendre en compte les proportions respectives des types de lèpre observés dans la région d'étude.RESUMEN
Se realizó un estudio con control de casos para establecer el efecto protector del BCG intradérmico contra la lepra y sus subtipos en Vietnam del Sur. Se seleccionaron 177 casos de los subtipos TT (38), BT (23), BB (51), BL (36), LL (22), e indeterminado (7). Cada caso se empató con dos controles en cuanto a edad, sexo, grupo étnico, estatus socioeconómico, y área de residencia. La probabilidad de un efecto protector del BCG varió de 0.44 (0.19-1.03) en el subtipo BB a 3.0 (0.24-37.5) en la lepra indeterminada, mientras que su valor global fue de 0.71 (0.45-1.10) para la lepra per se. Cuando se consideraron en conjunto todos los casos de lepra intermedia, se observó que el efecto protector del BCG tuvo una probabilidad de 0.48 (0.27 - 0.84). En las formas polares de la lepra, TT and LL, la probabilidad fue mayor de 1.0, con grandes intervalos de confianza. Es posible que el BCG induzca un cambio en la respuesta inmune hacia un mayor nivel de inmunidad celular. Cuando la vacuna de BCG se administra después de la infección primaria con Mycobacterium leprae, este cambio podría ser causa de aumento en la frecuencia de aparición de las formas moderadas y transitorias de la enfermedad. En la lepra TT, el BCG podría contribuir a la manifestación de las reacciones lepromatosas subclínicas preexistentes, mientras que en la LL estable, el BCG sería incapaz de inducir alguna forma de inmunidada protectora. Estos resultados confirman la importante variabilidad en la protección conferida por el BCG con respecto a las diferentes formas de la lepra, y puedan tener implicaciones importantes en el diseño de vacunas y en la interpretación de los resultados de los programas de vacunación ya que éstos deben tomar en cuenta la proporción de casos con las distintas formas de lepra en la región estudiada.Whereas BCG vaccine is given primarily to prevent tuberculosis, several prospective trials have revealed its protective efficacy against leprosy with a degree of protection varying from 20% to 80% (8). A recent additional trial performed in northern Malawi estimated this efficacy at about 50% (17). In these trials, nonlepromatous types of leprosy constituted the large majority of incident cases (93% in the Malawi study) and definitive conclusions concerning the protective effect of BCG against multibacillary (MB) leprosy were difficult to draw (7). During the last few years, the efficacy of BCG also was studied by several case-control studies (reviewed in Table 1) which provided consistent results with the prospective trials when considering all forms of leprosy ( leprosy per se ). A significant protective effect of BCG against leprosy per se, varying from 41% to 81%, was shown in northern Malawi (11), Brazil (20), Venezuela (6), and southern Malawi (2). This effect was estimated at 48% in Vietnam (1) and 20% in a large study in India (14), although not statistically significant. More conflicting results were reported when subtypes of leprosy were considered. In the two South American studies (6,20), the protection was found to be greater among MB patients (defined as LL, BL, BB, and also, for the Brazilian study, lepromin-negative indeterminate cases) than among paucibacillary (PB) patients. Conversely, in Vietnam (1), the protective effect was near zero for lepromatous leprosy (defined as LL and BL cases) whereas it was significant for nonlepromatous leprosy. In southern Malawi (11) the effect of BCG for MB leprosy (also defined as BL and LL) was found not significant, although estimated at 50%. In the Indian study (14), where there were no LL cases and very few BL cases, a significant protective effect was found for borderline leprosy (defined as BL, BB, BT cases).
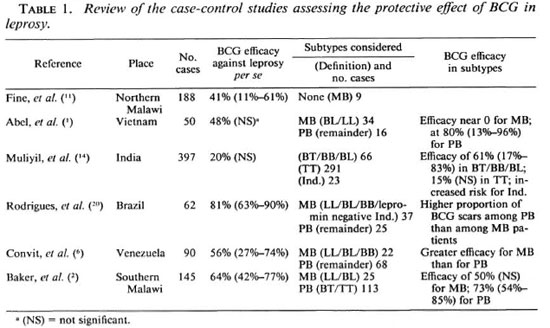
The aim of the present case-control study is to evaluate in southern Vietnam the variability of BCG efficacy according to leprosy clinical forms in a larger sample than our previous study (1).
METHODS
Study area. The study was conducted in Ho Chi Minh City, located in the south of Vietnam and representing a population of more than 4.4 million people. Leprosy control is the direct responsibility of administrative authorities at all levels, and the dermatology hospital is the center for the care of leprosy patients in the city area. Leprosy diagnosis, treatment and surveillance are done in accordance with the "Guide to Leprosy Control" by the World Health Organization (WHO). With the introduction of multidrug therapy (MDT), the prevalence had dropped in 1992 to just 0.2/1000 with an annual incidence rate of 6/100,000. Voluntary reporting of new patients was the most common mode of detection.
BCG vaccine was first introduced into the south of Vietnam in 1954 with mass vaccination campaigns that concentrated on both children 1-5 years old and children of the first-year class in primary schools (6-8 years old). Since 1964, newborns have been included in the official target population. According to available records, the vaccination program used Saigon Pasteur Institute liquid BCG and freeze-dried Canadian and Japanese BCG. However, it was not possible to assess the respective proportions of the BCG preparations that were actually used. The recommended intradermal dose was 0.1 ml in the left deltoid region. In 1972, vaccination coverages were 70% for children of the first-year class in public primary schools and 46% for newborns at public and private maternity hospitals. From 1986 to 1991, 83% of newborns in Ho Chi Minh City were vaccinated.
Study population. Cases were randomly collected from the file of leprosy patients under surveillance at the dermatology hospital of Ho Chi Minh City. Only patients younger than 25 years old were retained, because vaccination has been common only since 1964. A total of 177 cases were selected. They were subjected to a clinical and skin-smear examination and classified by experienced physicians using the Ridley and Jopling (18) classification into tuberculoid (TT), borderline tuberculoid (BT), borderline (BB), borderline lepromatous (BL), and lepromatous (LL) leprosy.
Two controls were matched with each case for age (±1 year), sex, ethnic group (Vietnamese/Chinese), socioeconomic status (three levels), and district area. Cases and controls were visited at their homes by a team consisting of a specialized physician, an auxiliary physician, and a community health worker to obtain the necessary information. Exposure to BCG was evaluated by looking for the typical scar over the deltoid region. BCG scars were read by three independent investigators who were blind to the study aim but not to the case or control status. Controls were examined to rule out any clinical evidence for leprosy.
Data were analyzed using the classical statistical methods developed for matched data with dichotomous exposures (3). The odds ratio (OR) and 95% confidence intervals (CI) were calculated to assess the protective effect of BCG against leprosy. Vaccine efficacy was computed as 1 minus the estimated relative risk (22); in a relatively rare disease, such as leprosy, the OR provides a good estimate of the relative risk.
RESULTS
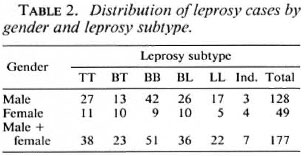
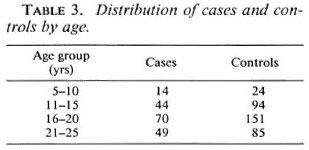
The distribution of leprosy cases by gender and leprosy subtype is shown in Table 2. As noted in other studies, the proportion of males was higher among the cases (72%) but there was no significant difference in the distribution of leprosy subtype by gender (x25df = 8.7, p > 0.1). The distribution of cases and controls according to age is shown in Table 3. The global proportion of positive BCG scars was 61% and 66.7% among cases and controls, respectively.
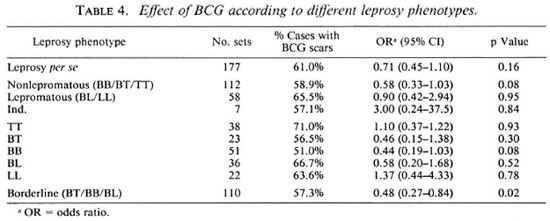
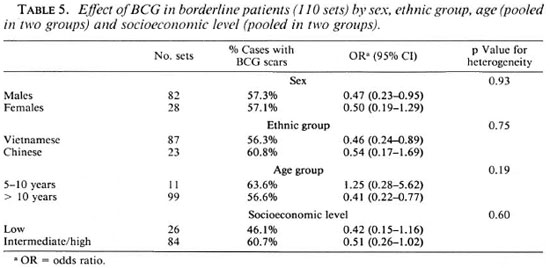
The results concerning the protective effect of BCG in different leprosy forms are presented in Table 4. When all forms of leprosy were pooled together (leprosy per se ), the protective effect (estimated at 29%) was not significant (x2ldf - 2, p = 0.16). In nonlepromatous patients (defined as BB/BT/ TT cases) the protective effect increased to 42%, but was still not significant (x2ldf = 3.2, p = 0.08). Since different results on the protective effect of BCG according to the leprosy form considered have been reported, the results for each of the leprosy subtypes are also presented in Table 4. In the three unstable borderline forms of the disease there was a trend in favor of a protective effect of BCG; this trend did not appear in the two polar forms TT and LL. Furthermore, when all borderline forms were pooled together, the protective effect of BCG was significant (x2ldf = 5.6, p < 0.02) and estimated at 52% (95% CI = 16%-73%). Among borderline patients, no significant heterogeneity of the BCG effect was found according to sex, ethnic group, age, or socioeconomic level (Table 5). However, it was interesting to note that the protective effect of BCG did not appear in the agegroup 5-10 years; none of these young patients had indeterminate leprosy. It is possible that some patients with early onset had been vaccinated after their infection with Mycobacterium leprae (during primary school), but there were not enough cases younger than 10 years old to assess this hypothesis.
DISCUSSION
The results of this study confirm the important variability in the protection offered by BCG with respect to the different types of leprosy, and indicate that assessing the protective effect of BCG using common classification, such as paucibacillary versus multibacillary or nonlepromatous versus Iepromatous leprosy, could be inappropriate. The odds ratio assessing the protective effect of BCG varies from 0.44 (95% CI = 0.19-1.03) in the BB subtype to 3.00 (0.24 - 37.5) in indeterminate leprosy; its overall value was 0.71 (0.45-1.10) for leprosy per se. When all borderline leprosy types were pooled, the protective effect of BCG was found significant with an odds ratio of 0.48 (0.27-0.84). In the polar forms of leprosy, TT and LL, the odds ratio was greater than 1 with large confidence intervals. BCG was already shown to provide a higher protection for borderline leprosy than for tuberculoid and indeterminate leprosy in India (14) and in New Guinea (21). The odds ratios obtained in the Indian study for indeterminate, tuberculoid, and borderline forms of leprosy were close to our results with values of 2.74 (0.84-8.95), 0.85 (0.59-1.22), and 0.39 (0.17-0.83), respectively; there was no case of Iepromatous leprosy in this study and no conclusion could be drawn with respect to this subtype. Moreover, an increased number of tuberculoid leprosy cases after BCG vaccination also has been described (23). As suggested by Muliyil, et al. (14), it is quite possible that one single intradermal injection of BCG could induce a boosting effect causing a shift in the immune response, and could reveal clinical immunopathological reactions in patients infected before the vaccination. Consequently, transient forms of leprosy, such as indeterminate forms, or subclinical forms of TT would be at higher risk after vaccination. Such a boosting effect with BCG vaccination has been observed in deer naturally or experimentally infected with mycobacteria (12). It would be important to know with accuracy the time of clinical diagnosis according to the time of vaccination, since increased risk to develop reactive forms could occur soon after the boosting effect produced by the BCG vaccination.
Two recent studies did conclude that BCG was able to give protection in MB leprosy (6,17). However, in the latter there were only 9 MB cases, and in the former MB cases grouped BB, BL, and LL patients (Table 1). Such a difference with our results could be associated with geographical variations in the immune spectrum of leprosy (9). The variability in the antigenic composition of the BCG preparations used in the present study does not appear to be a relevant factor to explain these discrepant results since a recent meta-analysis on the efficacy of BCG vaccine in tuberculosis showed that different BCG preparations and strains used in the same population gave similar levels of protection (4). Another hypothesis to account for this difference is the heterogeneity in the definition of the MB form with the pooling of unstable forms (borderline and unstable LL patients) with stable LL forms. The presence of unstable LL forms was demonstrated, for instance, in immunotherapy studies done with a mixture of killed M. leprae and BCG that reported lepromin conversions in two thirds of the Mitsudancgativc LL patients (5). A similar approach has been performed in an ongoing family study in Vietnam, where about one half of Mitsuda-negative LL patients did convert after one single injection of BCG (manuscript in preparation). It is thus quite possible that a) there is a great variation in the proportion of stable LL forms in different populations, and b) BCG can protect against unstable LL forms as against other unstable borderline forms; whereas it has no effect in stable LL leprosy. Under this hypothesis, the higher proportion of stable LL forms in Vietnam compared to South America or Africa can explain the absence of protection offered by the BCG vaccination in Vietnamese patients classified as lepromatous. Analogous experimental results have been reported already with armadillos vaccinated by BCG (13). In this study, BCG was able to convert the lepromin skin test in only 2 of 9 Florida armadillos, and a mixture of M. leprae and BCG did not give any better statistically significant results. Our study and others (14) confirm that leprosy cannot be considered as a simple dichotomous disease. According to the complex immune spectrum of leprosy, recommendations for strategics to control leprosy and future vaccine trials must evaluate separately these various immunoregulatcd disease subtypes.
Case-control studies appear as a cheap and useful tool to evaluate the efficacy of BCG in leprosy. The choice of controls in this kind of study has been discussed recently (15,16,19). In particular, it was stressed by Nishioka and Goulart (16) that the controls chosen in the Indian (14) and the Brazilian (20) studies might have had a lesser chance of developing leprosy than the cases, since cases were more likely to have had a household contact; the same can apply to the present study. However, as noted by Muliyil, et al. (14), a higher risk of infection due to household contacts can be a confounding factor only if it is also associated with BCG vaccination. In this type of study, it is certainly more important to choose controls with an opportunity to get BCG vaccination close to cases, rather than controls with an amount of exposure to M. leprae close to cases. In the present study the choice of matching controls for age, sex, ethnic group, socioeconomic status and district area was based on this principle.
Random misclassification could have occurred in the selection of cases and controls and in exposure ascertainment. In particular, misdiagnosis of indeterminate leprosy is possible without a skin biopsy, and exposure misclassification could be due to the failure to develop a recognizable scar for certain subjects vaccinated at birth (10). However, these phenomena would have the effect of moving the odds ratio closer to unity and, consequently, to underestimate the true effect of BCG. Furthermore, it was found in Ho Chi Minh City that the proportion of individuals vaccinated at birth who failed to present a scar was less than 5%, and, if this were the case, new vaccination was recommended for them.
In conclusion, our study finding that BCG offers about 52% of protection against borderline forms of leprosy in Vietnam supports the results of Muliyil, et al. (14) and the hypothesis that BCG can bring about a shift in the immune response to a higher level of cell-mediated immunity. However, when BCG vaccination is given after primary infection with M. leprae, this shift could be the cause of an increase in the risk of the occurrence of milder forms of the disease. When BCG efficacy was evaluated in other types of leprosy, no protective cfTcct was found in polar forms of the disease. In TT patients BCG might reinforce the preexisting subclinical immunopathological reactions, and in stable LL patients BCG might be unable to induce any protective form of immunity. Mass vaccination, such as the extended program of immunization that includes the BCG vaccine, would have varying effects within different geographic areas according to the relative proportion of stable or unstable BCG nonresponder individuals.
Acknowledgment. We are very grateful to all of the persons from the Dermato-Venereology Hospital of Ho Chi Minh City who have worked for this study: L. V. Hoa, P. H. Hai, P. A. Tuyet, H. V. Tu, N. L. D. Hien, M. T. Duong, N. T. Tien, V. H. Thai, V. C. Tarn, P. X. Khoa, N. H. Phu, C. T. B. Nam, T. T. T. Thuy, and T. Tinh. This work was supported in part by grant 491NS4 from I.N.S.E.R.M.
REFERENCES
1. ABEL, L., CUA, V. V., OBERTI, J., LAP, V. D., DUE, L. K., GROSSET, J. and LAGRANGE, P. H. Leprosy and BCG in southern Vietnam. (Letter) Lancet 335(1990)1536.
2. BAKER, D. M., NGUYEN-VAN-TAM, J. S. and SMITH, S. J. Protective efficacy of BCG vaccine against 538 International Journal of Leprosy 1994 leprosy in southern Malawi. Epidemiol. Infec. 111(1993)21-25.
3. BRESLOW, N. E. and DAY , N. E. Statistical Methods in Cancer Research. Vol. 1 -The Analysis of Case-Control Studies (IARC scientific publication no. 32). Lyon: IARC, 1980, pp. 162-189.
4. COLDITZ, G. A., BREWER, T. F., BARKEY, C. S., WILSON, M. E., BURDICK, E., FINEBERG, H. V. and MOSTELLER, F. Efficacy of BCG vaccine in the prevention of tuberculosis; meta-analysis of the published literature. JAMA 271(1994)698-702.
5. CONVIT, J. E., ARANZAZU, N., ULRICH, L. M., PINARDI, M. E., REYES, O. and ALVARADO, J. Immunotherapy with a mixture of Mycobacterium leprae and BCG in different forms of leprosy and in Mitsuda-ncgativccontacts. Int. J. Lepr. 50(1982)415-424.
6. CONVIT, J., SMITH, P. G., ZUNIGA, M., SAMPSON, C, ULRICH, M., PLATA, J. A., SILVA, J., MOLINA, J. and SALGADO , A. BCG vaccination protects against leprosy in Venezuela: a case-control study. Int. J. Lepr. 61(1993)185-191.
7. CRAWFORD, C. L. Leprosy vaccine. Nature 359(1992)100.
8. FINE , P. E. M. BCG vaccination against tuberculosis and leprosy. Br. Med. Bull. 44(1988)691-703.
9. FINE, P. E. M. Implications of genetics for epidemiology and control of leprosy. Phil. Trans. R. Soc. Lond. B 321(1988)365-376.
10. FINE, P. E. M., PÖNNIGHAUS, J. M. and MAINE, N. The distribution and implication of BCG scars in northern Malawi. Bull. WHO 67(1989)35-41.
11. FINE, P. E. M., PÖNNIGHAUS, J. M., MAINE, N., CLARKSON, J. A. and BLISS, L. Protective efficacy of BCG against leprosy in northern Malawi. Lancet 2(1986)409-502.
12. GRIFFIN, J. F. T. and BUCHAN, G. S. Vaccination against tuberculosis: is BCG more sinned against than sinner? Immunol. Cell Biol. 71(1993) 431-442.
13. JOB, C. K.., SANCHEZ, R. M., HUNT, R., TRUMAN, R. W. and HASTING, R. C. Armadillos (Dasypus novemcinctus) as a model to test antileprosy vaccines: a preliminary report. Int. J. Lepr. 61(1993)394-397.
14. MULIYIL, J., NELSON, K. E. and DIAMOND, E. L. Effect of BCG on the risk of leprosy in an endemic area: a case-control study. Int. J. Lepr. 59(1991)229-236.
15. MULIYIL, J., NELSON, K. E. and DIAMOND, E. L. Reply to the letter from Drs. Nishioka and Goulart. Int. J. Lepr. 61(1993)460-461.
16. NISHIOKA, S. E. and GOULART I. M. B. Assessment of BCG protective efficacy by case-control studies. Int. J. Lepr. 61(1993)459-460.
17. PONNIGHAUS, J. M., FINE, P. E. M., STERNE, J. A. C, WILSON, R. J., MSOSA, E., GRUER, P. J. K., JENKINS, P. A., LUCAS, S. B., LIOMBA, N. G. and BLISS , L. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet 339(1992)636-639.
18. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
19. RODRIGUES, M. L. O., ANDRADE, A. L. S. S., MARTELLI, C. M. T. and ZICKER, F. Reply to the letter from Drs. Nishioka and Goulart. Int. J. Lepr. 61(1993)461-462.
20. RODRIGUES, M. L. O., SILVA, S. A., NETO, J. C. A., DE ANDRADE, A. L. S. S., MARTELLI, C. M. T. and ZICKER , F. Protective effect of intradermal BCG against leprosy; a case-control study in central Brazil. Int. J. Lepr. 60(1992)335-339.
21. SCOTT, G. C, RUSSELL, D. A., BOUGHTON, C. R. and VINCIN , D. R. Untreated leprosy: probability for shifts in Ridley-Jopling classification; development of "flares" or disappearance of clinically apparent disease. Int. J. Lepr. 44(1976)110-122.
22. SMITH , P. G. Retrospective assessment of the effectiveness of BCG vaccination against tuberculosis using the case-control method. Tubercle 62(1982)23-35.
23. WADE, H. W. BCG-induced activations. Int. J. Lepr. 28(1960)179-181.
1. Pharm.D.; Section of Directives, Dermato-Venereology Hospital, Ho Chi Minh City, Vietnam.
2. M.D., Section of Directives, Dermato-Venereology Hospital, Ho Chi Minh City, Vietnam.
3. M.D., Ph.D., INSERM U194, Hôpital Pitie-Salpctricre, 91 Boulevard de l'Hôpital, 75013 Paris, France.
4. M.D., Ph.D., INSERM U65, Montpellier, France.
5. M.D., Ph.D., Department de Microbiologie, Hôpital St. Louis, Paris, France.
Reprint requests to Dr. Abel.
Received for publication on 9 May 1994.
Accepted for publication in revised form on 5 August 1994.