- Volume 62 , Number 4
- Page: 580–5
Calcium metabolism and its regulating hormones in patients with leprosy
ABSTRACT
Calcium metabolism was studied in 47 patients with borderline or lepromatous leprosy. Total and ionized calcium, phosphorus, creatinine, total alkaline phosphatase, parathyroid hormone (PTH), 25-hydroxy vitamin D [ 25(OH)D ] , and 1, 25dihydroxy vitamin D [ 1, 25(OH)2D ] were measured in serum; calcium and total hydroxyproline were determined in urine. Total subperiosteal diameter and medullar cavity diameter were measured on an X-ray of the hand of all patients.Average values were within normal ranges for all of the biochemical determinations. Total serum calcium was moderately below the normal range in eight patients but ionized calcium levels were within the normal ranges in all of the patients. Four patients, all of them with lepromatous leprosy, had levels of 1, 25(OH)2D higher than normal but none of them was hypercalcemic and PTH levels were within normal range. Although all values were within the normal ranges, lepromatous leprosy patients had lower total calcium, higher alkaline phosphatase, and higher urinary hydroxyproline than borderline leprosy patients (9.1 ± 0.4 vs 9.4 ± 0.3 mg%, p < 0.001; 10.3 ± 2.9 vs 7.4 ± 2.3 King-Armstrong units, p < 0.02 and 27.2 ± 12 vs 19.4 ± 5.6 mg/24 hr, p < 0.02, respectively). No differences were found between patients and controls in the average micrometric measurements of the second metacarpal bone but significant osteopenia was found in 19% of the patients.
The main finding of the present study in a representative sample of leprosy patients is that the average total serum calcium was in the lowest limit of the normal range, but the ionized serum calcium was in the middle of the normal range. Some patients had levels of 1, 25(OD)2D higher than normal but in none of them was the serum calcium increased. Therefore, it is possible that calcium homeostasis is normal in most patients with leprosy and only in exceptional patients would it be possible to find significant alterations.
RÉSUMÉ
Le metabolismo du calcium a été étudié chez 47 patients présentant une lepre borderline ou lépromateuse. Le calcium total et ionisé, le phosphore, la creatinine, les phosphatases alcalines totales, l'hormone parathyroídienne (HPT), la 25-hydroxy-vitamine D [ 25(OH)D ] , et la 1, 25-dihydroxy-vitamine D [ 1, 25(OH)2D ] ont été mesurés dans le sérum; le calcium et rhydroxyproline totale ont été mesurés dans les urines. Le diamètre sub-periostal total et le diamètre de la cavité médullaire ont été mesurés sur un cliché radiographique de la main de tous les patients.Les valeurs moyennes étaint dans les limites normales pour toutes les analyses biochmiques. Le calcium serique total était légèrement sous les valeurs normales chez huit patients, mais les taux de calcium ionisé étaient dans les limites normales chez tous les patients. Quatre patients, tous avec une lèpre lépromateuse, avaient des taux de [1, 25(OH)2D] supérieurs à la normale, mais aucun d'eux n'avait une hypercalcémie et les taux de HPT étaient dans les limites normales. Bien que toutes les valeurs étaient dans les limites normales, les patients lépromateux avaient des taux plus faibles de calcium, et plus élevés de phosphatases alcalines et d'hydroxyproline urinaire que les patients borderline (respectivement 9.1 ± 90.4 vs 9.4 ± 0.3 mg%, p < 0.001; 10.3 ± 2.9 vs 7.4 ± 2.3 unités King-Armstrong, p < 0.02 et 27.2 ± 12 vs 19.4 ± 5.6 mg/24 h, p < 0.02). Aucune différence n'a été observée entre les malades et les témoins dans les mesures micrométriques moyennes du deuxième métacarpien, mais une ostéopénie significative a été observée chez 19% des patients.
La découverte principale de cette étude dans un groupe représentatif de malades de la lèpre est que le calcium sérique total moyen était dans les limites inférieures de la normale, mais que le calcium sérique ionisé était au milieu des limites normales. Quelques malades avaient des taux de [1, 25(OH)2D] supérieurs à la normale, mais chez aucun d'eux le calcium sérique n'était augmenté. Il est donc possible que l'homeostasie calcique soit normale chez la plupart des patients lépreux, et que des modifications significatives ne puissent être trouvées qu'exceptionnellemnt chez des patients.
RESUMEN
Se estudió el metabolismo del calcio en 47 pacientes con lepra lepromatosa o lepromatosa subpolar. Se midieron en el suero las concentraciones de calcio ionizado, fósforo, creatinina, fosfalasa alcalina total, hormona paratiroidea (HPT), 25-hidroxi vitamina D [ 25(OH)D ] , y 1, 25-dihdroxi vitamina D [1, 25 (OH)2 D] . Los niveles de calcio y de hidroxiprolina se midieron en la orina. Además, se tomaron radiografías de la mano y en ellas se midieron el diámetro subperiostal total y el diámetro de la cavidad medular.Los valores promedio de todas las determinaciones bioquímicas estuvieron dentro de los rangos normales. La concentración de calcio total estuvo moderadamente por abajo de lo normal en 8 pacientes aunque los niveles de calcio ionizado estuvieron dentro de limites normales en todos los pacientes. Cuatro pacientes con lepra lepromatosa tuvieron niveles de 1, 25(OH)2D por arriba de lo normal pero ninguno de ellos fue hipercalcemico y los niveles de HPT estuvieron dentro del rango normal. Aunque todos los valores estuvieron dentro del rango normal, los pacientes con lepra lepromatosa tuvieron valores de calcio total más bajos, de fosfatasa alcalina más altos, y de hidroxiprolina urinaria también más altos que los pacientes con lepra subpolar (0.4 vs 9.4 ± 0.3 mg%, p < 0.001; 10.3 ± 2.9 vs 7.4 ± 2.3 unidades King-Armstrong, p < 0.02, y 27.2 ± 12 vs 19.4 ± 5.6 mg/24 h, p < 0.02, respectivamente). No se encontraron diferencias entre los pacientes y los controles en cuanto a las mediciones micrométricas promedio del segundo hueso metacarpal, aunque se encontró osteopenia significativa en el 19% de los pacientes.
El hallazgo principal del presente estudio en una muestra representativa de pacientes con lepra, fue que el promedio de calcio total en el suero estuvo en el límite más bajo del rango normal, aunque el nivel de calcio ionizado en suero estuvo en la parte media del rango normal. Algunos pacientes tuvieron niveles de 1, 25(OH)2D mayores de lo normal pero en ninguno de ellos se encontraron niveles elevados de calcio en el suero. Por lo tanto, parece ser que la homoestasis del calcio es normal en la mayoría de los pacientes con lepra, aunque pueden haber algunos casos excepcionales con alteraciones significativas.
Leprosy is a chronic granulomatous disease which can affect the skeleton, causing specific and nonspecific bone lesions (18,29). Although hypocalcemia has been the prevalent finding, at least in some leprosy centers located in India (19,20,25), hypercalcemia has been reported in five patients with leprosy (5,9,23,24). In four of them 1, 25-dihydroxy vitamin D3 [1, 25(OH)2D3] serum levels were augmented, suggesting that, as in other granulomatous diseases such as sareoidosis (2,3), the lepromatous granuloma might be an extrarenal source of 1, 25(OH)2D production.
In a recent review the number of leprosy patients around the world has been estimated to be 10 to 12 million, South America being one of the areas with the highest prevalence (16). Since most of the patients live in underdeveloped countries where serum calcium may not be routinely determined, Rizen and Singer (24) had suggested that the incidence of leprosy-associated hypercalcemia may be higher than it has been suspected.
In Argentina, the total number of leprosy patients is estimated to be 13,600, most of them living in the subtropical northeastern part of the country (4). The present study was undertaken in order to evaluate the frequency of alterations of calcium metabolism in patients with borderline or lepromatous disease who live in a leprosy center located in the area surrounding the city of Buenos Aires.
MATERIALS AND METHODS
Subjects. We studied 47 patients (31 men and 16 women) ranging in age from 18 to 75 (average age 48.6 ± 14.2 years) living in the Sommer Hospital, a leprosy center located in the outskirts of Buenos Aires, with a population that fluctuates between 400 and 500 patients. The 47 patients were included in the present study on the basis of their willingness to cooperate, and all of them gave verbal informed consent before the study. Patients with chronic renal failure or hepatic diseases as well as those who had been taking corticosteroids or who were not fully ambulatory during the 6 months prior to the study were excluded from the study.
Diagnosis and classification of the stage of the disease in all patients were assessed through nutritional, clinical, bacteriological, pathological and immunological features. The disease was judged to be active at the time of the study in 29 patients. The following data were recorded for each patient: age, sex, clinical form, activity and length of evolution of the disease, extent of the skin lesions, previous fractures and specific treatment for leprosy.
Forty-two patients had lepromatous leprosy and five had borderline leprosy. Skin involvement of the disease was diffuse in 23 patients and localized in 21. Twenty-six patients had had erythema nodosum leprosum (ENL); it was present at the time of the study in 13 patients. Peripheral neuritis was reported in 14 patients (in 6 patients at the time of the study), and arthritis was reported in 9 patients (in 2 ai the time of the study). Patients had received sulfone (39) rifampin (29), clofazimine (21) or thalidomide (6) as monotherapy or in combination.
Body mass index (BMI). The nutritional status of each patient was evaluated by Quetelet's index, also called body mass index (BMI). It was calculated as weight/height2 (kg/m2).
Biochemical analysis. Blood was drawn in the fasting state at the spring season and a 24-hr urine sample was collected on their usual daily diet. serum and urinary total calcium were measured by atomic absorption spectrophotometry (30); phosphorus by the colorimetric method of Taussky and Schorr (28) and creatinine by the colorimetric method of Taussky (27). Other serum determinations were: ionized calcium by a specific electrode (22), total alkaline phosphatase by the method of King and Armstrong (11), serum parathyroid hormone (PTH) concentration by radioimmunoassay (1), 25-hydroxy vitamin D [ 25(OH)D] and 1, 25(OH)2D concentrations by a solidphase chromatography radioreceptor follow by a competitive protein binding assay (8 and 21, 26, respectively). Total urinary hydroxyproline (THP) excretion was measured by the colorimetric method of Kivirikko, et at. (12) .
Radiological studies. All patients had an X-ray of the hands at a film-focus distance of 100 cm. The metacarpal cortical thickness was measured according to the principles established by Gam (6), The total subperiosteal diameter (T) and medullar cavity diameter (M) were measured to 0.02 mm with a scaler magnifier at the second metacarpal midshaft. Cortical thickness (C), medullar area (MA), total subperiosteal area (TA), cortical area (CA) and percent cortical area (PCA) were calculated on the basis of T and M by assuming that the metacarpal bone is circular in cross section. Each patient was compared to a normal subject of the same age and sex (14).
Statistical analysis. Individual results were analyzed, grouped and statistically evaluated. Data were expressed as mean ± standard deviation (S.E.M. ± S.D.) and evaluated by the Student's t test.
RESULTS
The BMI ranged between 19.2 and 36.5 (average 25.6 ± 3.8). Assuming a normal BMI of between 23 and 27, we found that 9% of the patients had a value below the normal range and 32% were above it.
Table 1 shows the average ± S.D. of the biochemical determinations made in all of the patients as well as the respective reference values. Average values were within the normal ranges for all of the biochemical parameters except for serum creatinine which was at the lower limit of the reference range. Total serum calcium was moderately below the normal range in eight patients (8.5 to 8.7 mg/dl), but serum levels of ionized calcium were within the normal range in all patients, including the eight with moderate hypocalcemia. serum phosphate was below normal in three patients. Serum PTH was higher than normal in one patient. Serum 25(OH)D was above the normal range in 3 patients and below in 6. Urinary determinations showed that 13 patients had a moderate hypocalciuria and in 6 of them the excretion was above the normal range; THP was above the reference value in 4 patients.
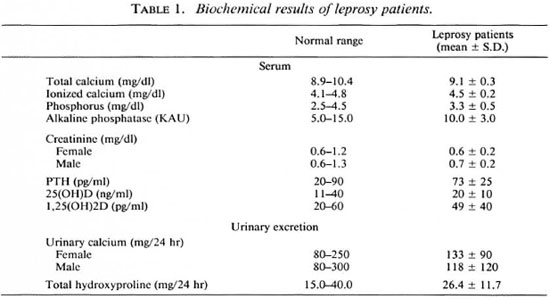
When the patients were divided into lepromatous and borderline leprosy, we found that the lepromatous patients had: a) lower total calcium (9.1 ± 0.4 vs. 9.4 ± 0.3 mg/ dl, p < 0.001); b) higher alkaline phosphatase [ 10.3 ± 29 vs. 7.4 ± 2.3 King Armstrong units (KAU), p < 0.02 ] and c) higher urinary THP excretion (27.2 ± 12 vs. 19.4 ± 5.6 mg/24 hr, p < 0.02), although all average values were within normal ranges.
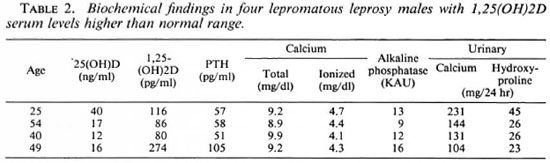
Four lepromatous leprosy patients had serum levels of 1, 25(OH)2D higher than normal (Table 2). None of the patients with increased 1, 25(OH)2D levels was hypercalcémie and their PTH levels were within the normal range (except for a minor elevation in one case) and without significant differences with the remaining patients.
The mean micrometric measurements of the medullary and cortical width of the second metacarpal bone were not different in patients and controls. However, the PCA was below 90%, indicating a significant osteopenia in 5 men and 3 women (19% of the cases). In those patients who underwent the study evaluations, X-rays of the hand were found to be abnormal in 19 (44%) of the 43 patients in whom the study was performed. The main findings were: a) claw deformity of the fingers, 11 cases; b) significant osteopenia, 8; c) bone cysts, 3; d) destruction of the last phalanx or the cortex, 6; e) tuft erosion, 2.
DISCUSSION
The main finding of the present study in a representative sample of leprosy patients is that the average total serum calcium is within the normal range although eight of them showed moderate total hypocalcemia (8.5 to 8.7 mg/dl). Furthermore, in all patients ionized serum calcium levels were definitively in the normal range, including those with moderate hypocalcemia. Thus, the frequent description of marked hypocalcemia (7.2 to 7.9 mg/dl) in leprosy patients in India (19,20,25) has not been confirmed. Our population was well nourished with an average BMI of 25.6, while the normal Indian patients had a BMI of 19.7 (25). Although BMI values of < 20 were regarded as indicative of underweight, factors such as diet, smoking and levels of physical activity confirmed that the Indian population, although relatively underweight, were healthy. It is thus not surprising that the range of acceptable values for Quetelet's index varies among communities (7).
There was a slight discrepancy between total and ionized serum calcium levels; while the former was in the normal low range, the latter was in the middle normal range. A similar finding has been reported recently by Vidal, et al. (31) in a leprosy center located in Rosario, Argentina. The authors observed that hypocalcemia in lepromatous leprosy could be related to an altered ability of serum proteins to bind calcium. These investigators found that at normal pH values, plasma proteins from lepromatous leprosy patients bind a smaller fraction of total plasma calcium than do controls. This pheomenon produces a normal concentration of ionized calcium which determines a normal parathyroid status.
Contrary to the prediction of Ryzen and Singer (24) that hypercalcemia could be a more frequent finding in leprosy if serum calcium were assessed routinely, none of our patients was hypercalcémie. Hypercalcemia and abnormal 1, 25(OH)2D concentration had been reported in four patients with borderline and lepromatous leprosy (5,9,23,24) . It has been recognized that several granulomatous diseases, such as sareoidosis (2,3), coccidioidomycosis (7), histoplasmosis (32), silicone implant (13) and eosinophilic granuloma (10), are associated with hypercalcemia and abnormal vitamin D metabolism. Hoffman, et al. (9) proposed that the presence of hypercalcemia in leprosy, as in other granulomatous disease (15), could be attributable to an ectopic production of calcitriol by the granulomas. Related to vitamin D metabolism, our findings suggest that the relation between 1, 25(OH)2D and leprosy is variable. Thus, only four patients exhibited levels of 1, 25(OH)2D higher than normal, but in disagreement with previous reports (5,9,23,24) none of the cases presented differences in their calcium levels, and PTH secretion was not suppressed.
We are not able to explain the normal biochemical finding in the four patients with elevated 1, 25(OH)2D serum levels. The elevation was moderate in three of them, therefore not being sufficient to overcome the other regulating mechanisms that keep serum calcium with the normal range. The abnormal secretion of 1, 25(OH)2D also could have been episodic or transient, lacking the persistence needed to alter mineral metabolism. Therefore, it seems that some patients with leprosy might have elevated 1, 25(OH)2D levels, but hypercalcemia seems to be an exceptional finding. None of the other biochemical determinations of mineral and bone metabolism were consistently altered. Therefore, it is possible to conclude that calcium homeostasis is normal in most patients with leprosy, and only in exceptional patients would it be possible to find significant alterations.
The X-ray of the hand showed significant osteopenia in approximately one fifth of the patients in relation to age- and sex-matched controls. Probably, diminished hand mobility, secondary to the neurological lesion, is the cause of the osteopenia. Thappa, et al. (29) observed that the osteoporotic bone changes were related to aging and severity of disability of the hands. The determination of the bone mineral density of the entire skeleton would permit one to assess if osteopenia is a localized phenomenon or if patients with leprosy have a general diminution of the bone mass.
REFERENCES
1. ARNAUD, CD., TSAO, H.S. and LITTLEDIKET, T. Radioimmunoassay of human parathyroid hormone in serum. J. Clin. Invest. 50(1971)21-31.
2. BARBOUR, G.L., COBURN, J.W., SLATOPOLSKY, E., NORMAN, A.W. and HORST, R.L. Hypercalcemia in an anephric patient with sareoidosis: evidence for extrarenal generation of 1, 25dihydroxy vitamin D. N. Engl. J. Med. 305(1981)440-443.
3. BELL, N.H., STERN, P.H., PANTZER. E., SINKA,T.K. and DE LUCA, H.F. Evidence that increased circulating 1, 25dihydroxy vitamin D is the probable cause for abnormal calcium metabolism in sareoidosis. J. Clin. Invest. 64(1979)218-225.
4. Boletín Epidemiólgico y Bibliográfico. Programa Nacional de Control de Lepra, Dirección de Epidemiología. Ministerio de Salud y Acción Social, (in press)
5. FRAZER, A.G., CROXSON, M.S. and ELLIS-PEGLER, R.B. Hypercalcemia and elevated 1, 25hydroxy vitamin D3 levels in a patient with multibacillary leprosy and a type 1 leprosy reaction. N. Z. Med. J. 100(1987)86.
6. GARN, S.M. The Earlier Gain and the Later Loss of Cortical Bone in Nutritional Perspective. Springfield, Illinois, U.S.A.: Charles C. Thomas Pub., 1970.
7. GIBSON, R. Anthropometric assessment of growth. In: Principles of Nutritional Assessment. New York: Oxford University Press, Inc., 1990, pp. 178-182.
8. HADDAD, J.G. and CHYU, K.J. Competitive protein-binding radioassay for 25hydroxycholccalciferol. J. Clin. Endocrinol. 33(1971)992-996.
9. HOFFMAN, V.N. and KORZENIOWSKI, O.M. Leprosy, hypercalcemia and elevated serum calcitriol levels. Ann. Intern. Med. 105(1986)890-891.
10. JURNEY, T.H. Hypercalcemia in patient with eosinophilic granuloma. Am. J. Med. 76(1984) 527-528.
11. KING, E. and ARMSTRONG, A.R. A convenient method for determining serum and bile phosphatase activity. Can. Med. Assoc. J. 31(1934)376-380.
12. KIVIRIKKO, K.I., LAITINEN, O. and PROCKOP, O.J. Modifications of a specific assay for hydroxyproline in urine. Ann. Biochem. 19(1967)249-257.
13. KOZENY, G.A., BARBATO, A.L., BANSAL, V.K., VERTUNO, L.L. and HANO, J.E. Hypercalcemia associated with silicone-induced granulomas. N. Engl. J. Med. 311(1984)1103-1105.
14. LABARRERE, C.A. and MAUTALEN, C.A. Parathyroid activity and bone formation. Metabolism 25(1976)135-138.
15. LEMANN, J., JR. and GRAY, R.W. Calcitriol, calcium and granulomatous disease. (Editorial) N. Engl. J. Med. 311(1984)1115-1117.
16. MEYERS, W.M. Leprosy. Dermatol. Clin. 10(1992)73-96.
17. PARKER, M.S., DOKOH, S., WOOLFENDEN, J.M. and BUCHSBAUM, H.W. Hypercalcemia in coccidioidomycosis. Am. J. Med. 76(1984)341-343.
18. PATERSON, D.E. and RAD, M. Bone changes in leprosy; their incidence, progress, prevention and arrest. Indian J. Lepr. 29(1961)393-422.
19. RAO, K.N., GUPTA, J.D., SEHGAL, V.N., CHAKR-ABARTI, A.K. and SAHA, K. Trace elements in the sera of leprosy spectrum. Indian J. Lepr. 57(1985)556-561.
20. RAO, K.N. and SAHA, K. Undernutrition in lepromatous leprosy. Part II. Altered levels of serum elements, their association with the disease and not with food deprivation. Lepr. Rev. 57(1986)311-316.
21. REINHARDT, T.A., HORST, R.L., ORF, J.W. and HOLLIS, B.W. A microassay for 1, 25dihydroxy vitamin D not requiring high performance liquid chromatography: application to clinical studies. J. Clin. Endocrinol. Metab. 58(1984)91-98.
22. Ross, J. W. Calcium-selective electrode with liquid ion-exchanger. Science 156(1967)1378.
23. RYZEN, E., REA, T. and SINGER F. Hypercalcemia and abnormal 1, 25dihydroxy vitamin D concentrations in leprosy. Am. J. Med. 84(1988)325-329.
24. RYZEN, E. and SINGER, F.R. Hypercalcemia in leprosy. areh. Intern. Med. 145(1985)1305-1306.
25. SAHA, K. and RAO, K.N. Undernutrition in lepromatous leprosy. V. Severe nutritional deficit in lepromatous patients co-infected with pulmonary tuberculosis. Eur. J. Clin. Nutr. 43(1989)117-128.
26. SHEPARD, R.M. and DE LUCA, H. Determination of vitamin D and its metabolites in plasma. Methods Enzymol. 67(1980)393-413.
27. TAUSSKY, H.H.p A microcolorimetric determination of creatinine in urine by the Jaffe reaction. J. Biol. Chem. 208(1954)853-860.
28. TAUSSKY, H.H. and SCHORR, E. Microcolorimetric method for determination of inorganic phosphorus. J. Biol. Chem. 202(1953)675-685.
29. THAPPA, D.M., SHARMA, V.K., KAUR, S. and SURI, S. Radiological changes in hands and feet in disabled leprosy patients: a clinico-radiological correlation. Indian J. Lepr. 64(1992)58-66.
30. TRUDEAU, D.L. and FREIER, E.F. Determination of calcium in urine and serum by atomic absorption spectrophotometry (AAS) Clin. Chem. 13(1967)101-114.
31. VIDAL, M.C., BOTTASSO, O., LEHRER, A. and PUCHE, R. Altered calcium-binding ability of plasma proteins as the cause of hypocalcemia in lepromatous leprosy. Int. J. Lepr. 61(1993)586-591.
32. WALKER, J.V., BARAN DM YAKUB, Y.N. and FREEMAN R.B. Histoplasmosis with hypercalcemia, renal failure, and papillary necrosis; confusion with sareoidosis. JAMA 237(1977)1350-1352.
1. M.D., Ph.D., Director; Seccion Ostcopatias Medicas, Hospital de Clinicas Jose de San Martin, University of Buenos Aires, Cordoba 2351, 1120 Buenos Aires, Argentina.
2. Ph.D.; Seccion Ostcopatias Medicas, Hospital de Clinicas Jose de San Martin, University of Buenos Aires, Cordoba 2351, 1120 Buenos Aires, Argentina.
3. M. D.; Seccion Ostcopatias Medicas, Hospital de Clinicas Jose de San Martin, University of Buenos Aires, Cordoba 2351, 1120 Buenos Aires, Argentina.
4. M.D.; Seccion Ostcopatias Medicas, Hospital de Clinicas Jose de San Martin, University of Buenos Aires, Cordoba 2351, 1120 Buenos Aires, Argentina.
5. Ph.D.; Seccion Ostcopatias Medicas, Hospital de Clinicas Jose de San Martin, University of Buenos Aires, Cordoba 2351, 1120 Buenos Aires, Argentina.
6. Biochemist, Seccion Ostcopatias Medicas, Hospital de Clinicas Jose de San Martin, University of Buenos Aires, Cordoba 2351, 1120 Buenos Aires, Argentina.
7. M.D.; Hospital Nacional Baldomero Sommer, General Rodriguez, Província de Buenos Aires, Argentina.
8. M.D., Hospital Nacional Baldomero Sommer, General Rodriguez, Província de Buenos Aires, Argentina.
Received for publication on 26 January 1994;
Accepted for publication in revised form on 7 July 1994.