- Volume 62 , Number 4
- Page: 586–93
Study of nerve conduction velocity, somatosensory-evoked potential and late responses (H-Reflex and F-Wave) of posterior tibial nerve in leprosy
ABSTRACT
The present study was conducted in 25 leprosy patients (of different age and sex) with or without clinical evidence of neuropathy. The diagnosis was confirmed by skin biopsy. A group of 15 age- and sexmatched, healthy persons also were studied for comparison and served as controls. Motor nerve conduction velocity (MNCV) was reduced in nine patients (36%) and sensory nerve conduction velocity (SNCV) was reduced in three patients (12%). Late responses (H-reflex and F-wave) were deranged in 16 patients (64%). Somatosensory evoked potential (SSEP) was deranged in 13 patients (52%). N7-N18 interpeak latency (PCT) was prolonged in two patients (8%); none showed prolongation of N18-N35 interpeak latency (CCT). We observed that nerve conduction velocity, late responses, and SSEPs were deranged in all types of leprosy, regardless of clinical evidence of neuropathy, and were more prominently affected in the tuberculoid (TT) type of leprosy. A study of late responses is more informative than conventional nerve conduction studies for the detection of early lesions of the nerves. The study of SSEP shows involvement of the peripheral part of the nervous system and complete sparing of the central part of the nervous system.RÉSUMÉ
L'étude présente été réalisée chez 25 malades de la lèpre (de différents âges et sexes) avec ou sans signes cliniques de neuropathie. Le diagnostic fut confirmé par biopsie cutanée. Un groupe de 15 personnes en bonne santé, appariés pour l'âge et le sexe ont également été étudiés en comparaison, et ont servie de témoins. La vitesse de conduction nerveuse motrice (VCNM) était diminuée chez neuf malades (36%) et la vitesse de conduction nerveuse sensorielle (VCNS) était diminuée chez, trois malades ( 12%). Les réponses tardives (réflexe H et onde F) étaient anormales chez 16 malades (64%). Le potentiel somatosensoricl évoqué (PSSE) était anormal chez. 13 malades (52%). Le temps de latence entre les pics N7 et N18 (PCT) était allongé chez. 2 malades (8%); aucun n'a montré un allongement de la latence inter-pics N18-N35 (CCT). Nous avons observé que la vitesse de conduction nerveuse, les réponses tardives et les PSSE étaient affectés dans tous les types de lèpre, qu'il y ait ou non des signes cliniques de neuropathie, et l'étaient surtout dans la lèpre de type tuberculoide (TT). Une étude des réponses tardives apporte plus d'informations que les études conventionnelles de conduction nerveuse pour la détection des lésions nerveuses précoces. L'étude des PSSE montre l'atteinte de la partie périphérique du système nerveux, alors que sa partie centrale reste complètement épargnée.RESUMEN
El presente estudio se realizó en 25 pacientes con lepra (de diferente edad y sexo), con o sin evidencias clínicas de neuropatía; el diagnóstico se confirmó en biopsias de piel. Como controles se incluyeron 15 personas sanas "empatadas" en edad y sexo. Se observó una reducción en la velocidad de conducción nerviosa motora en 9 pacientes (36%), y una reducción en la velocidad de conducción nerviosa sensorial en 3 pacientes (12%). Las respuestas tardías (reflejos-H y ondas-F) estuvieron afectadas en 16 pacientes (64%). El potencial somatosensorial evocado (PSSE) estuvo alterado en 13 pacientes (52%). En dos pacientes, el interpico de latencia N7-N18 estuvo prologado. Observamos que la velocidad de conducción nerviosa, las respuestas tardías, y los PSSE estuvieron alterados en todos tipos de los pacientes con lepra, independientemente de si presentaban o no, evidencias clínicas de neuropatía, y estuvieron afectados de manera más prominente en los pacientes con lepra tuberculoide (TT). Para la detección de lesiones tempranas de los nervios, el estudio de las respuestas tardías es más informativo que los estudios convencionales de conducción nerviosa. El estudio del PSSE, demuestra la afección de la parte periférica del sistema nervioso, sin afección de la parte central del mismo.Leprosy is primarily a diffuse peripheral neuropathy, and nerve involvement occurs prior to any clinical manifestation (1). Electrophysiological studies have revealed reduction in motor (MNCV) and sensory (SNCV) nerve conduction velocity in the ulnar, median, posterior tibial, common peroneal, sural and radial nerves in various types of leprosy (1,6,12,18,24,25). Nerve involvement in leprosy is segmental in nature (1,8), and observations concluded that nerve conduction studies arc of considerable value in the diagnosis and management of leprosy. Swift and coworkers (24) suggested that nerve conduction velocity and distal delay, especially for motor nerves, should be tested for the evaluation of the severity of leprosy. They also observed that MNCV and SNCV are equally affected in the neuropathy of leprosy. Dhand and coworkers (10) in 1988 studied phrenic nerve conduction velocity in leprosy patients and found that involvement of the diaphragm occurs even without clinical and radiological evidence of diaphragmatic dysfunction.
Late responses (H-reflex and F-wave) and somatosensory evoked potential (SSEP) studies in leprosy are not available in the literature. H-reflex and F-wave have been used to evaluate the function the nervous system (16,17,21). Significant prolongation of the minimal latency of late responses can be demonstrated at a time when conventional methods for studying motor and sensory conduction may not show an abnormality in an individual patient (17). Hence, it should be considered in the evaluation of nerve damage in the neuropathy of leprosy (4).
SSEPs have been used to determine the sensory conduction velocity in proximal segments of a nerve which arc not accessible by conventional methods (11,15). The scalprecorded SSEPs were absent or delayed in patients with a variety of polyneuropathies and mononeuropathies thus SSEPs may be helpful in segmental localization of nerve lesions in the neuropathy of leprosy.
MATERIALS AND METHODS
Patients
The present study was conducted on 25 freshly diagnosed patients (17 males and 8 females), ranging in age from 16 to 65 years, with different types of leprosy and before starting treatment. Detailed clinical and neurological examinations were carried out. The cases were classified according to Ridley and Jopling (20). There were 10 cases of tuberculoid (TT), 6 cases of borderline tuberculoid (BT), 6 cases of borderline lepromatous (BL), and 3 cases of lepromatous (LL) leprosy. Diagnosis was confirmed by skin biopsy. Other investigations included hemoglobin, total and differential leukocyte count, erythrocyte sedimentation rate, urinalysis, liver function tests, and skin smears for acid-fast bacilli (AFB). Patients with any other associated disease were not included in the study. A special search by history and biochemical analysis was made for conditions producing peripheral neuropathy, such as diabetes mellitus, collagen disorders, porphyria, chronic alcoholism, amyloidosis, etc. A detailed drug history also was taken and only those patients not taking any kind of medicine were incorporated into the study.
Controls
The control group was composed of 15 age- and sex-matched healthy persons (8 males, 7 females; age range 16 to 55 years) without any organic illness or drug treatment.
Electrophysiological studies
All of the recording sessions for SSEP, SNCV, MNCV, and late responses were performed in a shielded, partially soundproof, dimly lighted room. The apparatus used was Multibasis OTE Biomedica connected to a dot matrix printer.
SSEP and SNCV. The right posterior tibial nerve was stimulated percutaneously by a surface electrode at the ankle between the Achilles tendon and the medial malleolus. The stimulus strength was adjusted to produce a minimal twitch of the abductor hallucis muscle. The stimulating (cathode proximal) and recording Ag/AgC1 disk electrodes were fixed with collodion and filled with a conductive jelly to obtain an interelectrode resistance of 1-5 kilo-ohms. The active recording electrodes were attached at the popliteal fossa (pop. fossa), L-4 spine, C-7 spine, and at Cz (roughly corresponds foot area) of the 10-20 international system (13). A common reference electrode was attached at Fz of the 10-20 system. A belt ground electrode was wrapped around the leg. Each experiment was repeated 2 to 3 times and 200 trials were averaged following the stimulus. Onset sweeps were properly superimposed and filtered to separate timelocked from random activity. Peak and interpeak latencies were evaluated directly on the green fluorescent screen by a cursor. To get the SNCV of the distal segment of the posterior tibial nerve, the conduction time was measured between the ankle and pop. fossa by latency at the pop. fossa, and the distance between the ankle and the pop. fossa was divided by the conduction time. Thus,

MNCV. The motor nerve conduction velocity (MNCV) was obtained by stimulating the posterior tibial nerve at the ankle and the pop. fossa. The recording electrode was attached to the flexor hallucis bevis using surface electrodes (stimulating anode 2-3 cm proximal to the cathode, recording active electrode G1 being nearer to the cathode, indifferent electrode G2 over the tendon of the muscle) and the CAMP was recorded. Twenty to 30% supramaximal current was applied to obtain a biphasic response with initial negativity. The duration was measured from the onset to the final return at the baseline. The latency was measured from the stimulus artifact to the onset of the negative response. Thus,
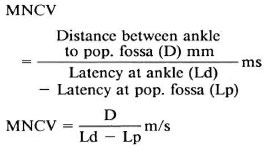
F-Wave. The F response of the abductor hallucis was elicited by supramaximal, percutaneous, antidromic stimulation at the ankle. The F-latency was measured as the minimal latency in a series of eight F-responses. With the help of F-wave latency (F) and M latency (M = initial muscle response provoked by electrical stimulus), the F-wave conduction velocity (FWCV) was (nerve conduction velocity of motor nerve from ankle to spinal cord) was measured. Thus,

H-Reflex. The H-reflex was recorded by placing the surface electrode below the lower margin of the medial gastrocnemius muscle. The posterior tibial nerve was stimulated in pop. fossa using surface, bipolar, stimulating electrodes with the cathode being proximal at the square wave pulses of one millisecond duration at a 5-second interval, and the voltage was increased gradually until a maximum reflex response was recorded. After eight consecutive responses, the response with minimal latency was used for measurement. Nerve conduction velocity of the proximal segment of the posterior tibial nerve is obtained by the H-reflex latency and M-latency as follows:

Statistical analysis. Neurophysiological data were compared and correlated by using the Student's t test. The upper limit of normal for all parameters measured was taken as the mean value of the controls + 3 S.D.
RESULTS
Results are given in Table 1 for the patients as a whole compared to the normal controls. There was a significant lengthening of the interpeak latencies between N7 (pop. fossa) and N18 (L5 area of the spine) in the patients, indicating a slowing of nerve conduction in the thigh in the peripheral nerves of the patients. Latencies were normal between the N18 (L5 area of the spinal cord) and N35 (sensory cerebral cortex of the brain) areas, indicating normal nerve conduction within the central nervous system in the leprosy patients.
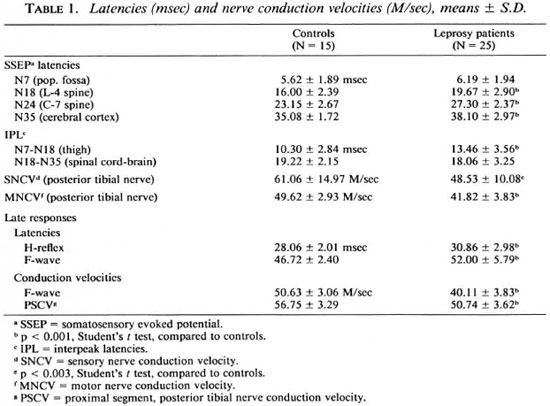
SNCV and MNCV of the posterior tibial nerves were decreased in the patients. H-reflex and F-wave latencies were increased, and F-wave conduction velocities and conduction velocities of the proximal segment of the posterior tibial nerve were decreased in the leprosy patients.
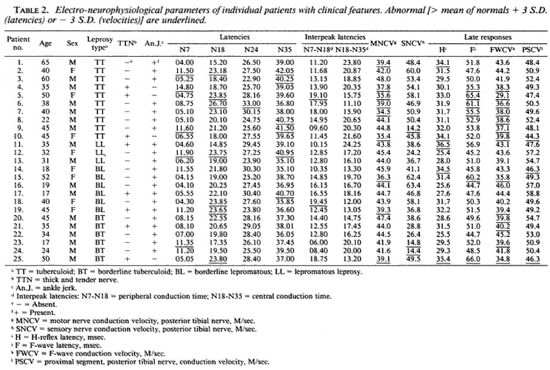
Data on individual patients arc given in Table 2. No clear pattern was seen in the clinical findings and the neurophysiological results. For example, six patients had prolonged latencies at N7 (more than the mean of the controls + 3 S.D.), namely, patients 2, 4, 9, 12, 14, and 23. One patient (no. 4) had a thick and tender nerve and ankle jerks were absent, but in the remaining five patients with prolonged latencies the nerves were not thickened and ankle jerks were present. The abnormal latencies were spread across the classifications of the disease with 3 having TT, 1 BT and 1 LL. Two patients (nos. 5 and 18) showed abnormally prolonged interpeak latencies between N7 (pop. fossa) and N18 (L5 area of the spinal cord) and a third patient (no. 25) almost exceeded 3 S.D. from the mean of the normal controls. Two of these had thickened and tender nerves and absent ankle jerks, but the third did not. These three patients had TT, BT, and BL disease. Fourteen of the patients (56%) showed slowing of the F-wave conduction velocities. Again, no particular pattern is apparent with the patients having disease of all types, with and without tender and enlarged nerves, and with and without ankle jerks.
Overall, some alteration in sensory nerve conduction (by SSEP, H-reflex, SNCV and PSCV) was seen in a total of 18 (72%) of the patients. Overall, motor nerve conduction (by MNCV, F-wave, and F-wave conduction velocity) was abnormal in 15 (60%) of the patients.
DISCUSSION
Neurophysiology is an important method to demonstrate early involvement of the nerves in leprosy in the presence of a clinically normal neurological examination. This has been used for the diagnosis of subclinical neuropathy in many conditions, for example, diabetic neuropathy (26), Guillain-Barre syndrome (16), alcoholic neuropathy (17), uremic neuropathy, etc. Study of the late response determines the changes in the proximal part of the peripheral nervous system (PNS) which is not easily accessible to routine neurophysiological studies. An abnormal late response indicates involvement of the PNS even when conventional NCV studies arc normal. It also has been shown that when parameters are deranged much earlier in many conditions, for example, in the Guillain-Barre syndrome prolongation of the late response may be the first, objective, recordable sign at an early stage of the disease when the diagnosis is difficult and CSF proteins are normal (16,19). However, it may be normal even in the presence of widespread neurological involvement, e.g., in Guillain-Barre syndrome (16).
The present research included the study of somatosensory evoked potential, the MNCV, SNCV, and late responses in all types of leprosy, and significant observations were made.
MNCV and SNCV
Although there are many hypotheses regarding the mode of affectation of the nerves and the mechanism of neural damage in leprosy, the basic lesion is segmental demyclination which could be recorded electrically as diminished conduction (8,24). Our study shows a significant slowing of the MNCV of the distal segment of the posterior tibial nerve (from the popliteal fossa to the ankle) in nine patients (36%); while SNCV was slowed in three patients. These observations are consistent with the observations made by other workers (8,24). It also shows that although clinically motor involvement is less common and less widespread than sensory involvement which always precedes paralysis in all types of leprosy, pathophysiological motor involvement is almost equal to sensory involvement (1).
In our study, eight patients had clinical involvement of the posterior tibial nerve, five of whom showed significantly diminished nerve conduction velocity, suggesting that motor and sensory nerve conduction may be normal in the diseased nerves. Similar observations also have been made by other workers (6,18,25). This is due to involvement of certain fascicles of the disease nerve with relatively little involvement of others. Since nerve conduction is calculated on the basis of the fastest conducting fibers only, this might still be normal in diseases nerves.
Fate responses
The H-reflex (24) is a monosynaptic reflex in which the afferent arc consists of group I a afferent fibers from muscle spindles and the efferent arc consists of alpha motor axons. The F-response is not a reflex but is produced by centrifugal discharges of a small percentage of motor neurons initiated by antidromic volleys in their axons; both afferent and efferent arcs consist of alpha motor axons (21). Recently late responses have been used widely to evaluate the function of the nervous system (16,17,21).
Significant alteration in late responses was observed in 16 patients (64%) as compared to diminished MNCV and SNCV (48% of cases). The H-reflex latency was delayed in five patients and the proximal segment conduction velocity determined by the H-reflex was significantly diminished in three patients. The F-wave latency was prolonged in 6 patients, and the FWCV was significantly diminished in 13 patients. It denotes the presence of nerve involvement even when conventional NCV are normal and clinically there is no evidence of nerve involvement. We could not find any study on late responses in leprosy for comparison. However, our observations are similar to those made by earlier workers that a significant prolongation of the late responses could be demonstrated at a time when conventional methods for studying MNCV and SNCV may not show an abnormality in an individual patient (16,21). This is due to the fact that the nerve conduction velocity represents a summation of activity of all axons within the nerve trunk while late response studies evaluate the function along an entire course of the motor axon so that abnormality in any particular segment can be detected and, secondly, the range of normal values for late responses are narrower compared with those for MNCV in different nerves, making it easier to detect mild abnormalities in individual patients (21).
SSEP
The most extensive studies of SSEP have been those following stimulation of the median nerve but only limited information is available for responses obtained with stimulation of the lower extremities (15). We performed this only to study the transmission of impulses through a greater length of the spinal cord. Posterior tibial nerve stimulation at the ankle evokes a traveling wave, N7, N18, N24 and N35 (11). Abnormal values of SSEP can be divided into a) those due to conduction in a peripheral nerve trunk and b) those due to conduction in the spinal cords.
Significant alteration in SSEP was observed in 13 patients (52%). N7 latency was significantly delayed in 5 patients (20%), N18 latency in 7 (28%), N24 latency in 1 (4%) and N35 latency in 6 patients (24%) as compared to slowed SNCV only in 3 cases. We could not find studies on SSEPs in leprosy patients but several studies in patients with a variety of polyneuropathies and mononeuropathies showed delayed or absent scalp-recorded SSEPs (11). This is because conventional SNCV deals with a more distal portion of the peripheral nerve, while SSEP allows assessment of the entire length of the somatosensory pathways. In leprosy there occurs segmental demyelination of the nerves (8) and more proximal involvement of the peripheral nerve could be undetected by conventional nerve conduction studies.
Interpeak latency N7-N18 (PCT) was prolonged in two patients (8%) while none showed prolonged interpeak latency N18-N35 (CCT). These observations indicate that peripheral conduction time (PCT) is prolonged but central conduction (CCT) remains normal, reflecting involvement of the peripheral part of the nervous system and absolute sparing of the spinal cord and thalamo-cortical pathways in leprosy.
Comparison between various types of leprosy
Our study shows that sensory nerve conduction (evaluated by SSEP, SNCV and H-reflex) was altered in 90% of tuberculoid leprosy patients, 67% of LL patients and 91% of borderline patients. Motor nerve conduction (evaluated by MNCV and F-wave) was altered in 80% of TT, 67% of LL and 60% of borderline leprosy patients. This is because the pathological process of neural involvement is different in different types of leprosy (3,22). In tuberculoid neuritis, which can occur without skin lesions (14), the powerful immune response of the host, represented by the focal masses of the epithelioid cells in the lesion, keeps the bacilli in abeyance, but the entire nerve parenchyma undergoes damage at the site of predilection so that there is extensive wallerian degeneration distal to these lesions (9). In lepromatous neuritis, on the other hand, the cell-mediated immune response is absent. Bacilli are present in abundance, especially in Schwann cells. There are usually extensive associated skin lesions, and there is more diffuse damage to both peripheral nerve myelin and axons (7).
Subclinical neuropathy
Our study shows that significant involvement was found on electro-neurophysiological testing of nerves in comparison to their clinical involvement. MNCV was slowed in 9 patients, SNCV in 3, late responses (H-reflex and F-wave) were deranged in 10, and SSEP in 8 patients whose posterior tibial nerve was not involved. This observation is similar to observations made by other workers in various types of neuropathy (5), showing subclinical involvement of the nerves in leprosy. This stage is the evolving phase of leprosy neuropathy, when derangement of neural functions (as detected by electrophysiological testing) occur probably due to subtle changes in intraneural blood vessels (2), disturbance of the delicate intraneural environment (23), or autoimmune neuritic reaction (7). At this stage the nerves are ultrastructurally and clinically normal since clinical involvement occurs only when ultrastructural changes in nerves are relatively advanced (22).
REFERENCES
1. ANTIA, N.H., PANDYA, S.S. and DASTUR, D.K . Nerves in arm in leprosy. I. Clinical, electrodiagnostic and operative aspects. Int. J. Lepr. 38(1970)12-29.
2. BODDINGIUS, J. Ultrastructural and histopathological studics on the blood-nerve barrier and peripheral barrier in leprosy neuropathy. Acta Neuropathol. (Berl.) 64(1984)282-296.
3. CARAYAN, A. Investigations on the pathophysiology of the nerve in leprosy. Int. J. Lepr. 39(1971)278-294.
4. CHOPRA, J.S., JAGANNATH, K. and SAIRLEY, I.M.S. Leprosy neuropathy. In: Recent Advances in Neuromuscular Diseases, 1991, pp. 7-16.
5. CRACCO, J., CASTELLS, S. and MARK, E. Conduction velocity in peripheral nerve and spinal afferent pathways in Juvenile diabetics. Neurology 30(1980)370-371.
6. DASH, M.S. A study of the conduction velocity of sensory fibers of the ulnar nerve in leprosy. Int. J. Lepr. 35(1967)460-469.
7. DASTUR, D.K. The peripheral neuropathy of leprosy. In: Symposium on Leprosy. Antia, N.H. and Dastur, D.K., cds. Bombay: Bombay University Press, 1967.
8. DASTUR, D.K. Infections of nervous system, part I. In: Handbook of Clinical Neurology. Editors Vinken, P.J., Bruyn, G.W. and Klawans, H. Amsterdam: North Holland Publishing, 33(1978)421-468.
9. DASTUR, D.K. and KABHOLKAR, A.S. Histochemistry of leprous nerves and skin lesions. J. Pathol. 113(1974)69-77.
10. DHAND, U.K., KUMAR, B., DHAND, R., CHOPRA, J.S. and KAUR, S. Phrenic nerve conduction in leprosy. Int. J. Lepr. 56(1988)389-393.
11. EISEN, A. and AMINOFF, M.J. Somatosensory evoked potential. In: Electrodiagnosis in Clinical Neurology. Aminoff, M.J., cd. New York: Churchill Livingstone Inc., 1986, pp. 535-574.
12. HACKETT, E.R., SHIPLELY, D.E. and LIVENGOOD, R. Motor nerve conduction velocity studies in patients with leprosy. Int. J. Lepr. 36(1968)282-287.
13. HARNER, P.F. and SANNIT, T. A review of international ten twenty system of electrode placement. Grass Instrument Co., Quincy, Mass., 1974. (Cited in Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice. 2nd edn. Kimura, J., cd. Philadelphia: F. A. Davis Company, 1989, 378-427.
14. JOPLING, W.H. and MORGAN HUGHES, J.A. Pure neural tuberculoid leprosy. Br. Med. J. 2(1965)799-800.
15. KIMURA, J. Somatosensory evoked potentials. In: Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice. 2nd edn. Philadelphia: F.A. Davis Company, 1989, pp. 378-427.
16. LACHMAN, T., SHAHANI, B.T. and YOUNG, R.R. Late responses as diagnostic aids in Landry-Guillain-Barre syndrome. Arch. Phys. Med Rehabil. 57(1976)600-606.
17. LEFEBVRE-D' AMOUR, M., SHAHANI, B.T., YOUNG, R.R. and BIRD, K.T. Importance of studying sural conduction and late responses in the evaluation of alcoholic subjects. Neurology 26(1976)368.
18. MCLEOD, J.G., HARGRAVE, J.C., WALSH, J.C., BOOTH, G.C. and GYE, R.S. Nerve conduction studies in leprosy. Int. J. Lepr. 43(1975)21-31.
19. ROPPER, A.H. and CHIPPA, K.H. Evoked potentials in Guillain-Barre syndrome. Neurology 36(1986)587-590.
20. RIDLEY, D.S. and JOPLING, W.H . Classification of leprosy according to immunity; a five-group system. Int J. Lepr. 34(1966)255-273.
21. SHAHANI, B.T. Late responses and silent period. In: Electrodiagnosis in Clinical Neurology. 2nd edn. Aminoff, M.J., cd. New York: Churchill Livingstone Inc., 1986, pp. 333-346.
22. SHETTY.V.S., ANTIA, N.H. and MEHTA,L.N. Study of the evolution of nerve damage in leprosy, part 1. Lepr. India 52(1980)5-18.
23. SHETTY, V.S. and ANTIA, N.H. Multiple axonal myelination in the experimental mouse leprosy model. Int. J. Lepr. 52(1984)249-251.
24. SWIFT, T.R., HACKETT, E.R., SHIPLEY, D.E. and MINER, K.M. The peroneal and tibial nerves in lepromatous leprosy: clinical and electrophysiologic observations. Int. J. Lepr. 41(1973)25-34.
25. VERGHESE, M., ITTIMANI, K.V., SATYANARAYAN, K.R., MATHAI, R. and BHAKTHAVIZIAM, C. A study of conduction velocity of ulnar and median nerves in leprosy. Int. J. Lepr. 38(1970)271-277.
26. WAGER, E.E., JR., and BUERGER, A.A. H-reflex latency and sensory conduction in normal and diabetic subjects. Arch. Phys. Med. Rehabil. 55(1974)126-132.
1. M.B.B.S., M.D.; Department of Medicine, S. P. Medical College and Associated Group of Hospitals, Bikaner 334001, India.
2. M.B.B.S., M.D., D. N., Department of Medicine, S. P. Medical College and Associated Group of Hospitals, Bikaner 334001, India.
Reprint requests to Dr. Bal Kishan Gupta, Code No. 1032, Khernada Mohella, Near old Power House, Police Line Road, Bikaner 334001, Rajasthan, India.
Received for publication on 5 January 1993;
Accepted for publication in revised form on 13 June 1994.