- Volume 62 , Number 4
- Page: 616–9
Mycobacterium lepraemurium itself does not interfere with the in vitro lymphoproliferation induced by concanavalin A
To the Editor:
Mycobacteria are microorganisms that penetrate the macrophages by active phagocytosis, and phagocytic killing is the only mechanism known for the "physiological" destruction of these bacteria (4). Macrophages, however, although efficient in general terms, have a limited capacity to destroy pathogenic mycobacteria such as Mycobacterium leprae and M. tuberculosis. The microbicidal activity of macrophages is highly potentiated by the effects of cellmediated immunity (CMI) through the several lymphokines produced, among which gamma-interferon (IFN- γ ) is of particular relevance. IFN- γ -activated macrophages exert clear deleterious effects on the leprosy bacillus (9).
Patients with lepromatous leprosy develop a specific loss of CMI to M. leprae antigens, and although the reason for this is still not well understood several tentative explanations have been put forward. Following infection, M. leprae cells are captured by polymorphonuclear leukocytes (PMNs) and macrophages. Despite the heavy bactericidal armory of PMNs, the combined circumstances of a short half-life of the cells and a highly resistant mycobacterial surface make their participation in controlling the infection not a determining event. Once inside the macrophages, the fate of M. leprae will depend upon the bactericidal efficiency of the cells tightly linked to the mechanisms of CMI. Macrophages of resistant individuals will destroy the bacilli, arresting the progression of the disease. At the same time, macrophages engaged in the destruction of M. leprae might eventually function as antigen-presenting cells (APCs). Through APCs, M. leprae interact with and activate M. leprae-reactive T cells, thus triggering the mechanisms of CMI.
Susceptible individuals lack either efficient macrophages or M. leprae-reactive T cells, which leads to bacillary multiplication and progression of the disease. It also has been suggested (10) that M. leprae might preferentially activate reactive, type-2 helper T cells (Th2). Activated Th2 cells would, in turn, abrogate the function of the cells responsible for CMI (Th1). It is also possible that direct contact of M. leprae or its products with M. leprae-reactive T cells would lead to their blockage, thus preventing their subsequent activation by mycobacterial antigens when they are appropriately presented by APCs.
In support of this possibility, some groups have shown that incubation of peripheral blood mononuclear cells (PBMCs) from healthy individuals, with M. leprae (13), or with mycobacterial products such as phenolic glycolipid-I (2), arabinomannan (1), or lepromin (5) renders the cells unable to proliferate in response to several stimuli, including mitogenic lectins such as concanavalin A (ConA) and phytohemagglutinin (PHA). PBMCs from patients with longlasting or untreated disease also are unable to proliferate in vitro in response to ConA and other stimuli, including lepromin and M. leprae (7).
Murine leprosy, on the other hand, is a disease of mice (and rats) that highly resembles human leprosy with regard to immunologic alterations. Because also in murine leprosy a specific loss of CMI to M. lepraemurium (MLM) arises, we decided to use the dimethylthiazol diphenyltetrazolium (MTT) colorimetric method of Mosmann (6) to explore the possibility that MLM might also interfere with the lymphocytes capability to proliferate in response to the stimulus with ConA.
Experimental. Adult, albino NIH mice were used as the source of spleen cells; NIH mice are at least as susceptible to the infection with M. lepraemurium as BALB/c mice. Cell suspensions were obtained according to standard protocols using Hanks' solution (In Vitro, Mexico) with 0.05% gelatin and 20 IU/ml heparin to prepare and wash the cells, and TC MEM (410-1100; GIBCO Labs, Grand Island, New York, U.S.A.) medium supplemented with 10% fetal calf serum to culture them. The TC MEM also contained gentamycin (100 µ g/ml), phenol red, and sodium bicarbonate to adjust the pH to 7.6. Cultures were set in quadruplicate in 24-well tissue culture plates; each set of four wells contained a) 1.5 million cells alone; b) the same amount of cells plus 2 µ l of ConA; c) the cells plus 15 x 106 M. lepraemurium; or d) a mixture of ConA and MLM, in 1.0 ml of TC MEM.
Lymphoproliferation was assessed by using the MTT colorimetric method of Mosmann (6), with minor modifications. Nonstimulated, ConA-activated, MLM-stimulated, and ConA + MLM-stimulated cultures were supplemented with 50 µ l of MTT (3- [4, 5-dimethylthiazol-2-yl] -2, 5 diphenyltetrazolium bromide) (M5655; Sigma Chemical Co., St. Louis, Missouri, U.S.A.) (7.5 mg MTT/ml PBS), reincubated at 37ºC for 120 min, and then harvested to measure the amount of reduced MTT. To do this, the entire contents of each culture well was repeatedly refluxed with a pipetor, collected and passed to a microcentrifuge tube, and then centrifuged at 350-400 X g for 5 min. After pouring off the supernatant and wiping off the inner rim of the tube, it was vortexed to loosen the cell pellet. Extraction of the reduced color in the pellet was done by adding 200 µ l of 0.04 N HC1 in isopropanol, followed by energetic vortexing. After centrifugation (1500 X g X 5 min), a 0.15-ml aliquot of each supernatant was placed in a clean ELISA plate and its absorbance was read by a densitometer set at 540 nm.
No statistical treatment of the data seemed to be necessary to draw definitive conclusions.
RESULTS AND DISCUSSION
Several independent experiments with a total of 12 mice led to the results shown in The Table (figures are mean MTT reduction values read at 650 nm). Compared to the resting, nonstimulated, spleen-cell cultures (0.064 ± 0.01, range 0.051 - 0.080), the cells stimulated with ConA showed variable but clearly higher MTT reduction values (0.247 ± 0.115, range 0.113- 0.484). Cultures stimulated with MLM behaved similar to resting cultures (0.063 ±0.010, range 0.048 - 0.078). Spleen cells stimulated with both ConA and MLM gave MTT reduction values similar to those values obtained with ConA (0.231 ± 0.118, range 0.097 - 0.473).
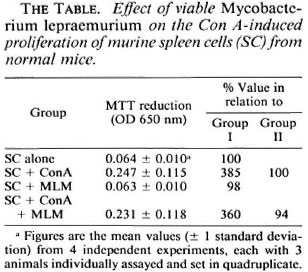
In summary, normal spleen cells responded well to the stimulus with ConA (385% of the response of resting cells) but showed no response to MLM whether viable (freshly prepared) or heat-inactivated (98% of the response of resting cells). Viable or nonviable MLM did not significantly modify the ConA-induced proliferative response of the cells (94% of the response of ConA activated cells, 360% of the response of resting cells; The Figure). Thus, under the conditions of the assay, the present results show (and confirm previous observations by Lemieux, et al. (3)) that the mere presence of MLM is not enough to affect the ability of murine T cells to proliferate in response to the mitogen ConA. The reported lack of (or depressed) reactivity to ConA of spleen cells from mice with long-lasting murine leprosy (3,8) must thus result from a series of rather complex interactions between the bacteria and the entire set of cells involved in the immunological handling of the bacillus. It is possible that the use of higher numbers of bacilli might lead to different results. However, considering that spleen cells constitute a heterogenous population with only a portion of the cells reactive to ConA, the ratio of 10 bacilli per cell used in the present study seemed to be appropriate to exclude or prevent unreal results attributable to an overdose of bacilli. The proposition that immune suppression in murine leprosy involves the participation of sets of cells and complex interactive mechanisms more than the mere presence of MLM is supported by our previous results on macrophage activation in vivo (12) . Mice infected with MLM develop a transient but marked state of macrophage activation (cell-mediated immunity) that is soon suppressed by as yet unraveled mechanisms when the number of bacilli in the lesions is still very low. At late stages of the infection however, higher numbers of bacilli may additionally contribute to the nonspecific immunosuppression exhibited by MLM-infected animals. The MTT test, although less sensitive than the radioactive method with tritiated thymidine, was sensitive enough to reliably assess the differences among the various systems investigated.
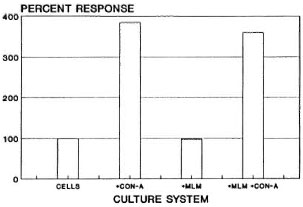
The figure. Proliferation of murine spleen cells (1.5 x 106 cells in 1.0 ml of TCM) in response to the stimulus with ConA (2 µ g), MLM (15 x 106 bacilli), or a mixture of MLM + ConA. Bars are the average percent responses from a total of 12 mice, each assayed individually in cultures set in quadruplicate.
- Oscar Rojas-Espinosa, Sc.D.
Ivonne Bonilla-Velázquez, Q.B.P.
Patricia Arce-Paredes, I.B.Q.
Departamento de Inmunología
Escuela Nacional de Ciencias Biológicas
Instituto Politécnico Nacional
Carpió y Plan de Ayala
Colonia Santo Tomás
11340 México, D.F., Mexico
Acknowledgment. P. Arce holds fellowships from COFAA(IPN) and IPN(BDA), O. Rojas-Espinosa is a fellow of COFAA(IPN), IPN(DBA) and SNI(Mex.). Funds were provided in part by the Dirección de Estudios de Posgrado e Investigación del IPN, México.
REFERENCES
1. ELLNER, J. J. and DANIEL, T. M. Immunosuppression by mycobacterial arabinomannan. Clin. Exp. Immunol. 35(1979)250-257.
2. KRISHNA, P., MISHRA, R. S. and NATH, I. Phenolic glycolipid-I of Mycobacterium leprae induces general suppression of in vitro concanavalin A responses unrelated to leprosy type. J. Exp. Med. 165(1987)239-244.
3. LEMIEUX, S., GOSSELIN, D., LUSIGNAN, Y. and TURCOTTE , R. Early accumulation of suppressor cell precursors in the spleen of Mycobacterium lepraemurium-infected mice and analysis of their in vi/ro-induced maturation. Clin. Exp. Immunol. 81(1990)116-122.
4. LOWRIE , D . B. The macrophage and mycobacterial infections. Trans. R. Soc. Trop. Med. 77(1983)646-655.
5. MEHRA, V., MASON, L. H., FIELDS, J. P. and BLOOM, B. R. Lepromin-induced suppressor cells in patients with leprosy.J. Immunol. 123(1987)1813-1817.
6. MOSMANN , T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65(1988)55-63.
7. NATH, I., CURTIS. J., SHARMA, A. K. and TALWAR, G. P. Circulating T-cell numbers and their mitogenic potential in leprosy-correlation with mycobacterial load. Clin. Exp. Immunol. 29(1977)393-400.
8. NAVALKAR, R. G., PATEL, P. J. and KANCHANA, M. V. Studies on immune response to Mycobacterium lepraemurium. Int. Arch. Allergy Appl. Immunol. 62(1980)423-431.
9. RAMASESH, N., ADAMS, L. B., FRANZBLAU, S. G. and KRAHENBUHL, J. L. Effects of activated macrophages on Mycobacterium leprae. Infect. Immun. 59(1991)2864-2869.
10. ROJAS-ESPINOSA, O. Active humoral immunity in the absence of cell-mediated immunity in murine leprosy: lastly an explanation. Int. J. Lepr. 62(1994)143-147.
11. ROJAS-ESPINOSA, O. and ESTRADA, P. S. Immunology of leprosy: cellular anergy to Mycobacterium leprae. Arch. Invest. Med. 20(1989)335-341.
12. ROJAS-ESPINOSA, O., VEGA, R., OLTRA, R. A., ARCE, P. P. and NUNEZ , A. Transitory macrophage activation in the granulomatous lesions of Mycobacterium lepraemurium-induced lepromatoid leprosy in the mouse. Int. J. Lepr. 56(1988)428-436.
13. Touw, J., STONER, G. L. and BELEHU, A. Effect of Mycobacterium leprae on lymphocyte proliferation: suppression of mitogen and antigen responses of human peripheral blood mononuclear cells. Clin. Exp. Immunol. 41(1980)397-405.