- Volume 62 , Number 3
- Page: 359–64
Activity of two doses of rifampin against Mycobacterium Leprae
ABSTRACT
In the course of a clinical trial designed to re-examine the bactericidal efficiency of 600-mg doses of rifampin (RMP) against Mycobacterium leprae, two doses of RMP, either 600 mg or 1200 mg, were administered 28 days apart to 29 previously untreated patients with lepromatous or borderline leprosy. Seven, 28, and 35 days after the start of the trial, skin biopsies were performed and immunologically normal mice were inoculated with 5 × 103 or 104 M. leprae in each hind foot pad. The patients assigned to the two regimens did not differ significantly in terms of sex, age, disease classification, bacterial index, or the concentration of M. leprae in the skin lesion biopsied for the inoculation of mice. The concentrations of organisms in the skinbiopsy specimens did not change significantly over the course of the trial among the patients, whether they were being treated by the first or the second regimen. The M. leprae recovered f rom specimens obtained f rom 21 of the patients, before beginning treatment, multiplied in a majority of the mice inoculated. The results of mouse inoculation confirmed the rapid bactericidal effects of RMP against M. leprae: a single dose of RMP rendered the organisms obtained f rom all but two of the patients incapable of multiplying in mice. No significant différence was demonstrated between the two regimens, nor was an additional effect of the second dose of RMP observed.RÉSUMÉ
Au cours d'un essai clinique destiné à réexaminer l'efficacité bactéricide de doses de 600 mg de rifampicine (RMP) vis-à-vis de Mycobacterium leprae, deux doses de RMP, soit 600 mg soit 1200 mg, ont été administrées à 28 jours d'intervalle à 29 patients non encore traités et présentant une lèpre lépromateuse ou borderline. Des biopsies cutanées ont été prélevées 7,28 et 35 jours après le début de l'essai, et on a inoculé des souris immunologiquement normales aves 5 × 103ou 10" M. leprae dans chaque coussinet plantaire des pattes postérieures. Les patients soumis aux deux régimes ne présentaient pas de différences significatives en ce qui concerne le sexe, l'âge, la classification de la maladie, l'index bactérien ou la concentration de M. leprae dans la lésion cutanée biopsiée pour inoculation à la souris. Les concentrations d'organismes dans les biopsies n'ont pas changé de manière significative parmi les patients au cours de l'essai thérapeutique, qu'ils soient traités par le premier ou le second régime thérapeutique. Les M. leprae retrouvés à partir des spécimens obtenus chez 21 des patients avant le début du traitement se multipliaient dans une majorité des souris inoculées. Les résultats de l'inoculation des souris ont confirmé les effets bactéricides rapides de la RMP vis-à-vis de M. leprae: une dose unique a rendu les organismes obtenus à partir de tous les patients, sauf deux, incapables de se multiplier chez la souris. Aucune différence significative n'a été mise en évidence entre les deux régimes, et on n'a pas observé d'effet additionnel de la seconde dose de RMP.RESUMEN
Se diseñó un ensayo clínico para re-examinar la eficiencia bactericida de 600 mg de rifampina (RMP) contra Mycobacterium leprae en 29 pacientes con lepra lepromatosa o lepra intermedia sin tratamiento. Se administraron dos dosis de 600 ó de 1200 mg de RMP separadas por 28 días. A los 7, a los 28, y a los 35 días de iniciado el tratamiento se tomaron biopsias de piel para preparar suspensiones de bacilos. Con este material (5 × 103 o 104 M. leprae) se inocularon las almohadillas plantares traseras de ratones inmunologicamente competentes. Los pacientes asignados a los dos regímenes de tratamiento no difirieron significativamente en cuanto a sexo, edad, clasificación de su enfermedad, índice bacteriano, o concentración de M. leprae en la piel biopsiada. Las concentraciones de organismos en los especímenes de piel no cambiaron significativamente durante el curso del ensayo y fue lo mismo para los pacientes que recibieron cualquiera de los dos esquemas de tratamiento. Las micobacterias recuperadas de los especímenes de piel de 21 pacientes antes del tratamiento se multiplicaron en la mayoría de los ratones inoculados. Los resultados de la inoculación de ratones confirman el rápido efecto bactericida de la RMP contra M. leprae: con sólo dos excepciones, una sola dosis de RMP hizo a los microorganismos obtenidos de todos los pacientes incapaces de multiplicarse en los ratones. No se observaron diferencias significativas entre los dos esquemas de tratamiento y tampoco se observó un efecto adicional de la segunda dosis de RMP.Rifampin (RMP), the most important single component of the combined drug regimens for both paucibacillary and multibacillary leprosy recommended by the Study Group on Chemotherapy of Leprosy for Control Programs, that was convened by the World Health Organization in 1981 (4), is administered monthly in an oral dosage of 600 mg. In a small clinical trial (1) the drug had been shown to be rapidly bactericidal against Mycobacterium leprae when administered as monotherapy in a single oral dose of 600 mg. In fact, this dosage was not shown to be less active than when the drug was administered in single doses of 900, 1200 or 1500 mg.
The WHO Study Group recognized the importance of fully supervised drug administration, particularly of RMP, in order to better ensure patient compliance. It was clear that drug administration could be fully supervised only if the drug were active when administered intermittently. In addition, intermittent administration of RMP offered the advantages of reducing the cost and potential toxicity of RMP-containing drug regimens. That single doses of RMP were rapidly bactericidal suggested that the drug might indeed be active when administered intermittently. However, the findings of the earlier trial had not been confirmed by a second clinical trial. Therefore, the decision was made to undertake a trial in which a single dose of RMP was to be administered as monotherapy to previously untreated patients with multibacillary leprosy; the efficacy of the treatment to be measured by inoculation of mice with M. leprae recovered by biopsy from the patients' lesions at 7 and 28 days after the dose.
It was also decided to administer a second dose immediately after the 28-day biopsy, and to perform an additional biopsy and inoculate mice 7 days after the second dose of RMP.
MATERIALS AND METHODS
Beginning in 1986, 29 previously untreated patients (21 males and 8 females between 18 and 60 years of age) with lepromatous or borderline leprosy, who came for treatment to the outpatient clinic of the Research Department of the Institut Marchoux and who agreed to participate in the trial, were randomly allocated to treatment with a single supervised dose of RMP, either 600 or 1200 mg, on day 1 and again on day 29, together with a daily multivitamin tablet.
The patients were hospitalized for the duration of the trial. Upon admission to the trial, a complete physical examination was performed, and smears were obtained from both earlobes and from four other sites for determination of their bacterial index (BI). In addition, two biopsy specimens were obtained from prominently involved areas of skin; one specimen was fixed and prepared for histopathologic study, the second served as the source of M. leprae for inoculation of mice. The number of organisms/g of tissue was measured, and 10 mice were inoculated with 5 x 103 organisms in each hind foot pad. A urine specimen was examined for the presence of dapsone, and a hematologic examination and measurements of liver and kidney function were performed on a sample of blood. Classification of the patients' disease was determined on the basis of both clinical and histopathological findings.
In the course of the trial, a complete physical examination was performed at intervals of 1 week, and also whenever required by the patients' complaints. On the basis of the findings during clinical examinations, a "clinical score" was calculated modified from the THELEP Standard Protocol (6), as shown in Table 1. Similarly, a "disability score" was calculated as described in the report of the WHO Study Group (5) (Table 2). In addition, urine specimens were examined weekly for the presence of dapsone.
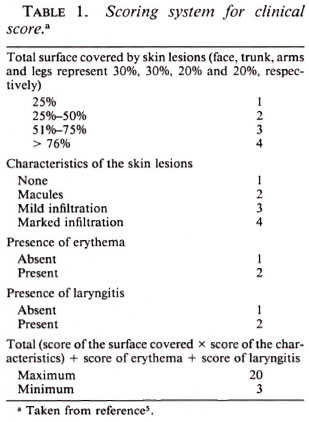
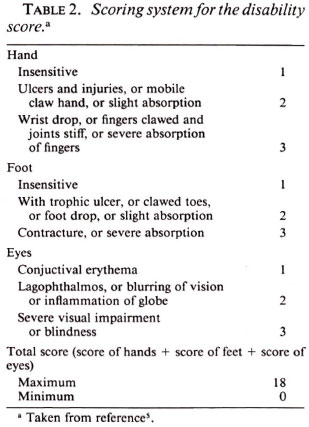
Seven, 28 and 35 days after the start of the trial, skin biopsies were performed and mice inoculated: 8 with 5 x 103 M. leprae into each hind foot pad, and 8 with 104 organisms per hind foot pad. At 12 months after inoculation, at least five mice in each group were sacrificed, and harvests of M. leprae were carried out from the pooled hind foot pads of each mouse by Rees' method (2).
The data resulting from this trial were analyzed by means of Fisher's exact probability calculation and the Mann-Whitney U test, both nonparametric tests of statistical significance (3).
RESULTS
The characteristics of the patients recruited into this trial are summarized in Table 3. As is usual among patients with multibacillary (MB) leprosy who present spontaneously for treatment at the Institut Marchoux, more than twice as many males as females were recruited. The proportion of female patients did not differ significantly between the two regimens (p = 0.30). Similarly, the patients assigned to the two regimens did not differ significantly either in terms of disease classification (p = 0.29), age (p = 0.57), or the interval between the onset of disease recognized by the patient and his first consultation with a physician (p = 0.56). Finally, the two groups of patients did not differ with respect to either BI (p = 0.41) or the concentration of M. leprae in the skin lesion biopsied for inoculation in mice (p = 0.17).

The results obtained by enumerating M. leprae in the biopsy specimens and inoculating mice with standard numbers of the organisms are summarized in Table 4. As shown by the data in this table, the concentration of organisms in the skin-biopsy specimens did not change significantly over the course of the trial among patients treated by either regimen.
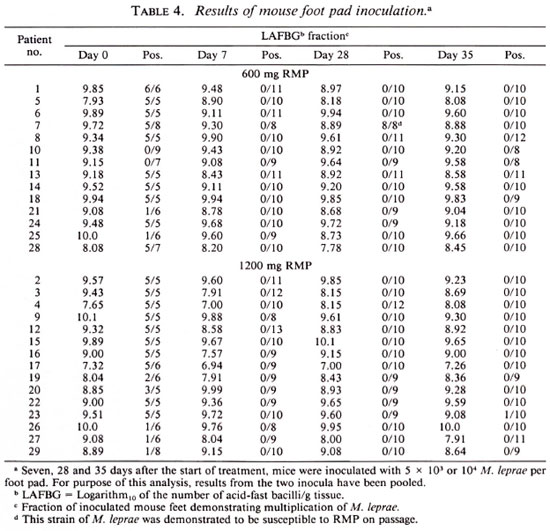
The M. leprae recovered from the specimens obtained before beginning treatment of all but two patients (patient nos. 10 and 11, both in the group treated with 600 mg of RMP) multiplied in mice. The organisms of six patients (patients nos. 21 and 25, treated by 600 mg of RMP and patients nos. 19, 26, 27 and 29, treated by 1200 mg of RMP) multiplied in only a minority of the inoculated mice. These data indicate that the proportions of viable M. leprae in these patients' pretreatment specimens were very small. By contrast, the organisms recovered before treatment from 21 patients (10 patients treated by 600 mg of RMP, and 11 patients treated with 1200 mg of RMP) were found to have multiplied in most if not all of the mice subjected to harvest, indicating that the proportion of viable organisms was no smaller than 1:1000.
The results of mouse inoculation (Table 4) confirm the rapid bactericidal effects of RMP against M. leprae. Considering only the 21 patients whose M. leprae infected the majority of mice inoculated before the start of treatment, and considering the mice inoculated with 5 x 103 organisms together with those inoculated with 104 M. leprae, a single dose of RMP rendered the organisms obtained from almost all of the patients incapable of multiplying in mice, i.e., the dose of RMP reduced the proportion of viable organisms to less than 1:1000. With only two exceptions-the 28-day specimen obtained from patient no. 7 and the 35-day specimen obtained from patient no. 23 - the M. leprae recovered from the 63 biopsy specimens obtained after treatment was begun uniformly failed to multiply in mice. No significant difference can be demonstrated between the two regimens. Moreover, no additional effect of the second dose of RMP was observed.
Because the analyses of the urine specimens uniformly failed to demonstrate evidence of dapsone intake, it appears unlikely that the surreptitious intake of dapsone contributed to these results.
As shown by the data of Table 5, in which the clinical and disability scores of the patients of the two treatment groups are presented, both regimens were very effective, and no significant difference between the two regimens was demonstrated in terms of clinical improvement.
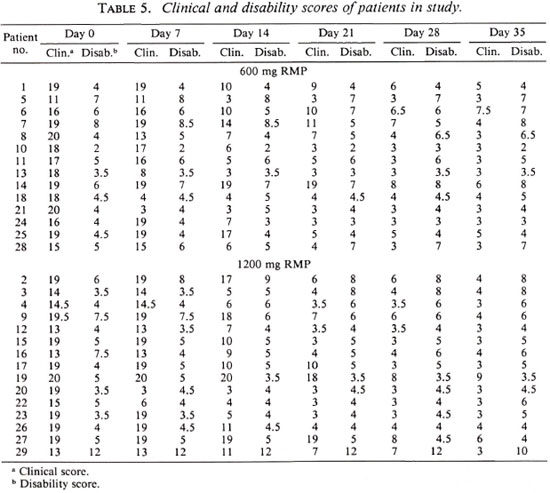
The patients of both treatment groups demonstrated remarkable improvement over the course of the 35-day trial despite the fact that the entire treatment consisted of two doses of RMP, the second administered 28 days after the first. In fact, as is clear from the data in Table 5, much of the clinical improvement may be attributed to the first dose of RMP. On the other hand, no change in the disability score was observed among the patients treated by either regimen during the 35 days of the trial.
DISCUSSION
The purpose of this trial was to re-examine the bactericidal efficiency of two doses of RMP, either 600 or 1200 mg, administered 28 days apart. An earlier trial among patients with previously untreated MB leprosy, carried out in San Francisco, had demonstrated (1) that the M. leprae recovered from 10 biopsy specimens obtained from five patients between 3 to 9 days after a single dose of 1200 mg of RMP failed to infect mice; whereas the organisms recovered from 1 of a total of 12 specimens obtained from six patients 3 to 9 days after a single dose of 600 mg were capable of multiplying in normal mice that had been inoculated with 5 x 103 M. leprae in a hind foot pad.
Thus, RMP administered in a single, 600mg dose was not demonstrated to be less effective than when the drug was administered in a single dose of 1200 mg (p > 0.50).
In confirmation of the results of an earlier trial, RMP proved to be fully effective in almost all patients in the sense that single doses rendered the patients' M. leprae incapable of multiplying in mice inoculated with 5 or 10 x 103 organisms per foot pad as early as 7 days after the dose.
In the current study, considering for the moment only the first dose of RMP, data are available from 20 specimens, representing those 10 patients treated with 600 mg of RMP whose M. leprae multiplied in the majority of mice inoculated before the start of treatment, and 24 specimens, representing the 12 patients treated with 1200 mg of RMP whose organisms obtained before treatment multiplied in the majority of mice. No difference was demonstrated between the two doses (p > 0.4). Because a single dose was virtually fully effective, no additional effects of the second dose could have been demonstrated.
These results demonstrate that a 600-mg dose of RMP, such as is employed in the WHO Study Group regimen, is maximally effective so far as can be demonstrated by inoculation of normal mice. On the other hand, the trial does not provide information with respect to the interval between "pulses" of RMP, or to the number of doses required for "cure."
Acknowledgment. This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. The authors also wish to express their gratitude to Professor L. Levy for his assistance in editing this manuscript.
REFERENCES
1. Levy, L., Shepard, C. C. and Fasal, F. The bactericidal effect of rifampin on M. leprae in man: a) single doses of 600, 900 and 1200 mg; and b) daily doses of 300 mg. Int. J. Lepr. 44(1976)183-187.
2. Rees, R. J. W. Limited multiplication of acid-fast bacilli in the foot-pads of mice inoculated with Mycobacterium leprae. Br. J. Exp. Pathol. 45(1964)207-218.
3. Siegel, S. Non-Parametric Statistics for the Behavioral Sciences. New York: McGraw-Hill, 1956.
4. Who Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
5. Who Study Group. Epidemiology of leprosy in relation to control. Geneva: World Health Organization, 1985. Tech. Rep. Ser. 716.
6. World Health Organization. Standard protocol for chemotherapy trials in lepromatous leprosy. Geneva: World Health Organization, 1977. TDR/ SWG-THELEP (l)/77.3., Annex 1.
1. M.D.; Institut Marchoux, B.P. 251, Bamako, Mali.
2. D.Sc; Institut Marchoux, B.P. 251, Bamako, Mali.
3. M.D.; Institut Marchoux, B.P. 251, Bamako, Mali.
Reprint requests to Dr. Traore.
Received for publication on 19 April 1994
Accepted for publication on 6 June 1994.