- Volume 62 , Number 3
- Page: 365–73
Long-term evaluation of immune status in leprosy patients undergoing multiple drug therapy
ABSTRACT
A long-term survey of leprosy patients of all clinical types, starting at the time of diagnosis, was carried out to monitor clinical, bacteriological and immunological parameters at regular intervals during multiple drug therapy (MDT). The patients were assigned to two groups for treatment following WHO guidelines: paucibacillary (PB) and multibacillary (MB). Immunoglobulin levels, specific antibodies, skin-test responses to different soluble mycobacterial antigens (new tuberculins), and in vitro proliferative responses to mitogens and to antigens were measured during treatment, as were clinical changes, the bacterial index, and clinical improvement. No exact relations between disease activity and IgM antibody levels, both IgM immunoglobulin and specific IgM antibody to a species-specific antigen (ND-O-BSA), could be seen for MB patients. Changes in in vitro cell-mediated immunity and skin-test response seemed to be more directly related to the bacterial load and could reflect the improvement of bacteriological and clinical parameters during MDT.RÉSUMÉ
On a réalisé une étude à long terme de malades de la lèpre de tous les types cliniques, débutant au moment du diagnostic, afin de surveiller des paramètres cliniques, bactériologiques et immunologiques à intervalles réguliers au cours de la polychimiothérapie (PCT). Les patients ont été répartis en deux groupes pour le traitement, selon les recommandations de l'OMS: paucibacillaires (PB) et multibacillaircs (MB). Les taux d'immunoglobulines, les anticorps spécifiques, les réponses aux tests cutanés à différents antigènes mycobactériens solubles (nouvelles tuberculines), ainsi que les réponses prolifërativcs in vitro à des mitogènes et antigènes ont été mesurés durant le traitement, tout comme les modifications clinques, l'indice bactérien et l'amélioration clinique. On n'a pas observé pour les patients MB de relation précise entre l'activité de la maladie et les taux d'anticorps IgM, d'immunoglobulines IgM et d'anticorps IgM spécifiques à un antigène spécifique d'espèce (ND-O-BSA). Les changements dans l'immunité cellulaire in vitro et dans la réponse au test cutané semblaient être plus directement liés à la charge bactérienne et pourraient refléter l'amélioration des paramètres bactériologiques et cliniques durant la PCT.RESUMEN
Se investigaron las características clínicas, bacteriológicas e inmunológicas de pacientes con lepra paucibacilar o multibacilar en tratamiento con poliquimioterapia. El estudio se hizo a intervalos regulares durante un período prolongado de tiempo y las mediciones incluyeron los niveles de inmunoglobulinas y de anticuerpos específicos, las respuestas dérmicas a diferentes antígenos micobacterianos solubles (nuevas tuberculinas), las respuestas linfoproliferativas inducidas in vitro con antígenos y mitógenos, así como los cambios clínicos, el índice bacteriológico, y la mejoría clínica. En los pacientes multibacilares no se observaron relaciones exactas entre la actividad de la enfermedad y los niveles de anticuerpos IgM contra el antígeno específico ND-O-BSA. Los cambios en la inmunidad celular demostrada in vitro y la respuestas intradermicas estuvieron relacionados con la carga bacteriana y pudieron reflejar la mejoría en los parámetros bacteriológico y clínico durante la poliquimioterapia.Multiple drug therapy (MDT) has been recommended by the World Health Organization (WHO) during the last decades for the treatment of leprosy and has been very effective. One of the difficulties in treatment is to find a satisfactory quantitative measurement of a patient's progress toward a successful outcome. Currently used methods of assessing response to drug therapy are still subjective: clinical observation and bacterial index (BI). Many studies have reported the changes in antibody levels of patients to Mycobacterium leprae sonicated antigens and specific antigens, including the phenolic glycolipid (PGL-I) and the specific disaccharide (ND-O-BSA), during MDT (1,7,13,14), but long-term studies on the changes in cell-mediated responses have not been reported.
In this study, a group of leprosy patients in Hanoi, Vietnam, was followed from the time of diagnosis throughout the period of MDT to monitor clinical, bacteriological and immunological parameters at regular intervals. Our aim was to document the immunological status before and during MDT for up to 3 years, and to find out whether a change in clinical presentation due to MDT was associated with immunological changes, in particular in cell-mediated immunity. Immunoglobulin levels, specific antibodies, skin-test responses and in vitro proliferative responses to mitogens and to antigens were measured at regular intervals to gain an overall picture of the immune status of the patients before, during and after MDT.
MATERIALS AND METHODS
Patients
The patient group consisted of 25 newly diagnosed, untreated patients with different types of leprosy, classified on clinical and bacterial grounds according to the Ridley-Jopling scale (17). They were all from the area around Hanoi in the northern part of Vietnam (detailed information on the patients is summarized in The Table).
Control blood was usually taken from students of the Medical Faculty of Hanoi University and, occasionally, from laboratory workers.
Blood collection
Ten ml of blood was collected before treatment (T0) and at regular intervals of 3 to 6 months for serological and cellular immunological tests. At the same time, the patients were examined clinically and bacteriologically (bacterial index; BI) and skin tests were done.
Treatment
The two regimens recommended by the WHO Study Group (25) were used for treatment of paucibacillary (PB) and multibacillary (MB) leprosy (24):
Regimen I for PB leprosy: Rifampin 600 mg once monthly for 6 months (supervised) plus dapsone 100 mg (1-2 mg/kg body weight) daily (self-administered) for 6 months.
Regimen II for MB leprosy: Rifampin 600 mg once monthly (supervised); 50 mg of clofazimine daily and 300 mg once monthly (supervised) and 50 mg of dapsonc daily (self-administered). The duration of treatment was at least 2 years and was continued, whenever possible, until smears became negative.
Immunological tests
Serological assays. Total immunoglobulin levels were determined in a standard Mancini test, using class-specific precipitating antisera to IgG, IgM and IgA (Central Laboratory of the Blood Transfusion Service, Amsterdam, The Netherlands).
Specific IgM antibodies of M. leprae were assayed in an ELISA using a semi-synthetic disaccharide antigen coupled to bovine serum albumin (BSA) (ND-O-BSA, kindly provided by WHO from the TDR/IMMLEP Programme) according to the WHO protocol (6), briefly as follows: U-bottom Immulon II plates (Dynatech Laboratories, Alexandria, Virginia, U.S. A) were coated with ND-O-BSA or BSA alone (0.1 mg/ml in carbonate-bicarbonate buffer, pH 9.6) for 18 hr at 37ºC. After washing with phosphate buffered saline (PBS) containing 0.05% Tween 20 (PBST), and blocking with BSA (5% in PBS, pH 7.2) for 1 hr at 37ºC, the test serum was added, diluted 1/300 in PBST + 10% normal goat serum. Anti-human IgM conjugated with horseradish peroxidase (Cappel, Organon Teknika, West Chester, Pennsylvania, U.S.A.; diluted 1:2000) was used to detect bound antibodies, followed by the addition of TMB-substrate [Sigma Chemical Co., St. Louis, Missouri, U.S.A.; 12 mg of 3,3'-5,5'-tetramethylbenzidine (TMB) in 5 ml of ethanol added to 15 ml of 0.1 M citrate/phosphate buffer, pH 5.0; H 2 0 2 added to a final concentration of 0.015%] and reading of optical density (OD) at 450 nm. The specific IgM antibody level was expressed as the OD value in ND-O-BSA wells after subtracting the OD value in parallel wells containing only BSA.
For comparison with the ND-O-BSA-ELISA results, a particle agglutination test measuring antibodies to PGL-I of M. leprae was also done, according to the protocol supplied by the manufacturers of the kit (Fujirebio Inc., Tokyo, Japan).
Cellular immunity
Skin testing. A panel of four mycobacterial antigens (new tuberculins) prepared from M. tuberculosis (tuberculin), M. leprae (leprosin A), M. vaccae and M. scrofulaceum were used as skin-test reagents; they were prepared by the Mycobacteria Laboratory of the National Institute of Hygiene and Epidemiology, Hanoi, according to a standard protocol (11). For skin testing, 0.2 mg (0.1 ml) of each antigen was injected intradermally into the volar surfaces of the forearms. Each person was tested with four antigens, two on each arm. Reactions were read at 72 hr by measuring the longitudinal and transverse diameters of induration in mm. A diameter of induration of 2 mm or more was considered positive.
In vitro lymphocyte stimulation test (LST). In vitro responsiveness of peripheral blood lymphocytes to mitogens and antigens was measured by the 3H-thymidine incorporation method, as previously described (22). Briefly, mononuclear cells isolated by centrifugation over Ficoll-Hypaque were seeded into wells of round-bottom microtiter plates (Greiner, Germany) at 5 or 7.5 x 104 cells/well in Iscove's medium (GIBCO, Grand Island, New York, U.S.A.) supplemented with 10% inactivated human AB serum (HS). To these was added 50 /d of antigen on day 0 or, on day 3, mitogen, diluted in Iscove's medium + human serum. All cultures were set up in triplicate. The results are expressed as counts per minute (cpm) ± standard deviation (S.D.) or in some cases as a stimulation index (SI), i.e., the ratio of the mean counts in treated cultures to that in untreated control cultures.
In some cases, cytokine-rich supernatants were added to cultures; they were prepared from control cultures of lymphocytes from healthy donors in medium containing 10 µ g/ml PHA, harvested at 48 hr.
The significance of differences and the relations between results at different times or from different groups were tested with the Wilcoxon rank sum test and Spearman's rank correlation.
RESULTS
Changes in mean total immunoglobulin levels of leprosy patients during MDT
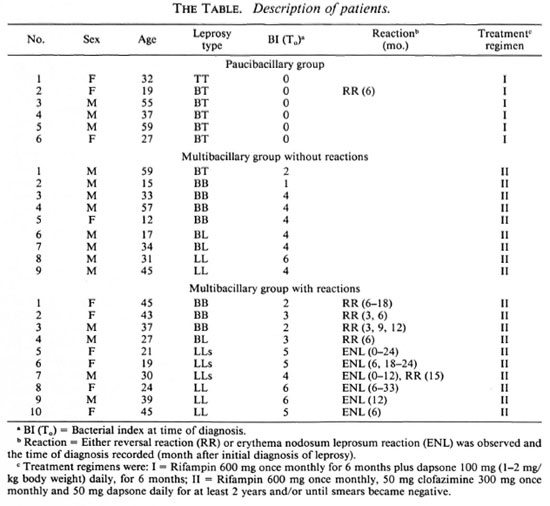
The mean immunoglobulin level in the sera of leprosy patients during treatment was compared with that of normal individuals (Figure 1). Although the variation among individuals was great, in all patients total IgG declined significantly between T0 and 24 months (p < 0.001) to a level comparable with the mean value of IgG of healthy controls. The total IgM level remained higher than the mean value for normal individuals throughout the whole period (p < 0.002). No significant differences were found in IgA levels between the leprosy patients and the healthy controls.
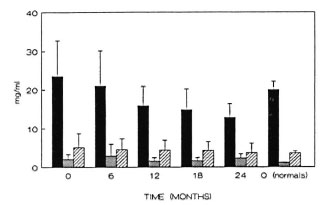
Fig. 1. Total immunoglobulin levels of leprosypatients on MDT. The Mancini technique was used tomeasure total immunoglobulin levels, shown in mg/ml, averages (± standard deviation, S.D.) of all patients at different time points during treatment. ■ =IgG:  = IgM;M
= IgM;M  = IgA.
= IgA.
IgM specific antibodies to ND-O-BSA and BI
The presence of IgM antibody to the disaccharide antigen specific for M. leprae (ND) is often considered to be an indication of the presence of bacteria in the patient. The changes in mean IgM antibody levels to ND-O-BSA in paucibacillary (PB) and multibacillary (MB) leprosy patient groups were compared with the changes in BI. As can be seen in Figure 2, the average BI decreased gradually in MB patients after 2 years (p < 0.02). During the same period, there was no decrease of IgM antibody levels against ND in the MB group, which were higher than in PB patients. A decrease in the level of IgM antibodies to ND could be detected in the PB group after 1 year of treatment (p < 0.01).
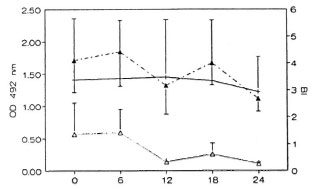
Fig. 2. Levels of antibodies to ND-O-BSA andchanges in 131 in MB patients on MDT. The averageBI (--▲--) for the MB patients is shown along with theaverage antibody levels (± S.D.) in the ND-O-BSAELISA for both MB (- +- ) and PB (- -) patients.
-) patients.
Comparison of detection of M. leprae-specific IgM antibodies by ELISA and a particle agglutination test
Although the ELISA is the most commonly used test for antibodies to the surface antigens of M. leprae, other tests are available. We compared the results of the ELISA described above with those of a commercially available particle agglutination test. The correlation between the values for IgM-specific antibodies to the sugar terminals measured by ELISA and IgM-specific antibodies to PGL-I measured by the particle agglutination test was good (Spearman's r = 0.59, p < 0.001).
Skin-test responsiveness in MB patients
The local cellular responses of leprosy patients to mycobacterial antigens were measured by skin testing at intervals during the treatment period. The results for the MB patients are shown in Figure 3. It can be seen that the percentage of skin-test responders to the tuberculin derived from M. scrofulaceum rose to 40% during the first 6 months in MB cases, but declined again to the original level of 10%-15% by 12 months. Responses to M. leprae, tuberculin, and the M. vaccae antigen also increased but to a lower level in the first 6 months. All reactions decreased and remained at the same low level throughout the remaining period of treatment. The range of reactivity (assessed by the mean diameter of induration) was not the same for all antigens. Reactions were strongest to scrofulin (T0, 7.5 ± 3.5 mm; T6, 5 ± 2.5 mm), then to vaccin and tuberculin (T0, 5.3 ± 0.6 mm; T6, 4-5 ± 3 mm), while those to leprosin were just above the minimum of 2 (up to 2.7 mm).
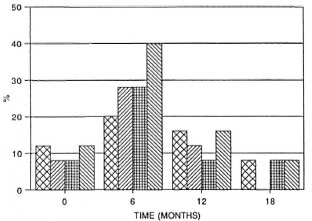
Fig 3. Skin-test responsiveness to inycobacterialantigens in MB patients on MDT. Reactions were readat 72 hr by measuring the longitudinal and transversediameters of induration in min; a diameter of induration of 2 mm or more was considered positive. Bars represent the percentage of responders among thosetested at each time point to each of tour skin-test antigens. Total number of patients tested at each point varied between 18 and 25 ± S.E.M. for each patient group is shown.  = tuberculin;
= tuberculin;  = leprosin;
= leprosin;  = M. vaccae ;
= M. vaccae ; 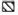 = M. scrofulaceum .
= M. scrofulaceum .
In vitro lymphoproliferative response to mitogens
When the in vitro proliferative responses to mitogens were compared between PB and MB groups, no significant differences were found. The data were therefore pooled for presentation in Figure 4. The changes in response during the treatment period were similar for the three mitogens tested, phytohemagglutinin A (PHA), concanavalin A (ConA) and pokeweed mitogen (PWM). They were all low in the beginning (p < 0.01) but reached control levels during the first 2 years. PHA and ConA responses decreased again 3 years after initiation of treatment (p < 0.01), but this decrease was not observed for PWM response.
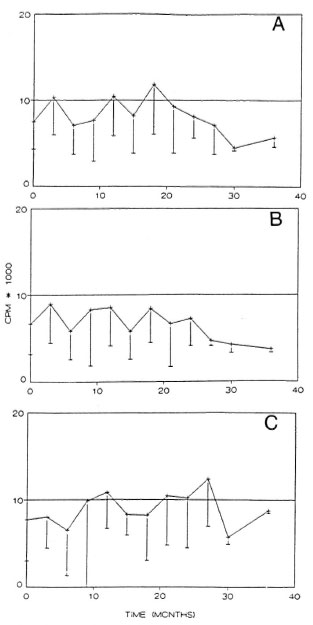
Fig. 4. In vitro proliferative response to mitogensduring MDT. The average of responses by all patientsto three mitogens is shown: A = response to PHA, B= response to ConA, C = response to PHA. Horizontalline = average response of healthy controls.
In vitro lymphoproliferative responses to M. leprae sonicated antigens
Although great individual variations were encountered, the mean in vitro response to sonicated whole M. leprae antigen increased from the starting level by 18 months in both PB and MB patient groups (p < 0.01); for the MB group, the responses decreased again after that time point (Fig. 5).
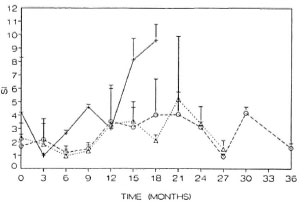
Fig. 5. In vitro proliferative response to sonicated M. leprae antigen during MDT, showing the responsesof PB (- + - ) patients and MB patients with (MB+)( A ) or without (MB) ( 0 ) reactionsduring treatment expressed as stimulation index (SI).
Reactions during treatment and cell-mediated immune responses during reactions
Several of the patients in the study suffered reactions during MDT: ENL occurred in 6 of 8 LL/LLs cases, while reversal reactions were observed in 5 of 16 borderline patients; one LLs patient had both ENL and reversal reaction (RR) (The Table). The earliest point at which RR was noted was 3 months after treatment (2/5); the others were between 3 and 9 months. One patient had several episodes, while others had very long episodes. ENL reactions appeared at the beginning of treatment (2/6) or at 6 months (3/6). They lasted as little as 1 month (2/6) or much longer, up to 27 months (3/6). Half of the patients with reactions had only one episode; the others had two or three episodes.
In some cases, blood samples for culture were taken from patients undergoing reactions. When the percentage of positive responders in the lymphocyte stimulation test (LST) against M. leprae sonicates in such patients was compared, 27.8% had positive responses (SI > 2) during ENL and 80% during RR. In comparison, there were 36.7% positive responders among patients who did not develop reactions. In Figure 6, the changes in response to sonicated M. leprae antigens of three patients who had reactions are shown. In the BB patient during an RR episode the response to M. leprae increased (p < 0.001); this increase could be amplified further with the addition of cytokine-rich supernatants (Fig. 6A). The LLs patient had during the course of his disease two types of reactions, ENL and RR (Fig. 6B). Similar to the BB patient, his response increased during ENL but decreased again during a subsequent episode of RR. The third patient shown had subpolar LL, with ENL over a long period (24 months); in this case the changes were less pronounced than in the other two cases (Fig. 6C).
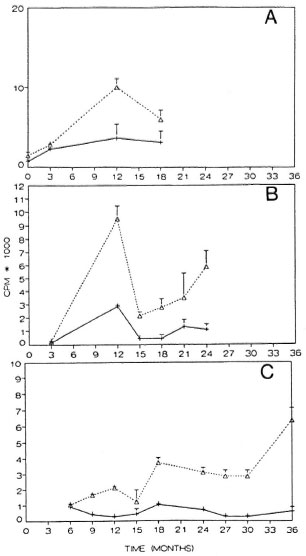
Fig. 6. Cell-mediated immune response and reactions during MDT. Response to sonicated M. leprae antigen is shown for three patients who had reactionsduring treatment: A = BB patient with a reversal re-action at the time points 3, 9, and 12 months; B = LLs patient with ENL during 0-12 months and RR at 15months; C = LLs patient with ENL from 0-24 months. Responses are shown as cpm at different time points in the presence (+sup) (·····A·····) or absence (- sup)(- + - ) of added cytokine-rich supernatant.
DISCUSSION
Multiple drug therapy has long been known to give satisfying rates of clinical, bacteriological and histopathological improvement in leprosy patients. In this study, different parameters of immunological status, especially cell-mediated immunity, before and during MDT were followed up to find out whether a clinical and bacteriological improvement by MDT would be accompanied by a change in immunological responses. A variety of techniques was used to measure immunoglobulin levels and specific antibody levels in the patients' sera over the course of 2 years of treatment. A chronic elevated level of total IgM was noted during at least the first 2 years of treatment. Antibody levels to ND-O-BSA in MB patients were high at the beginning of treatment, compared with PB patients, and did not change, while the BI decreased during the period of study. These results are in agreement with those reported by Lyons, et ai, Melsom, et al. and Truman, et al. (12,13,23), but are different from what was found by Moudgil, et al. (15) and Roche, et al. (18). The persistence of high levels of these antibodies might be explained by the enormous load of antigen present in MB cases, which might be expected to take many years to clear. The test to detect antibody to ND-O-BSA was specific for IgM; there is a possibility that patients began to produce IgG antibodies which could either replace IgM antibodies or compete with them in the ELISA, resulting in an apparent decrease in IgM antibodies to ND-O-BSA. The decrease in total IgG levels observed might be explained by the suppression of responses by M. leprae antigens later in the treatment period.
The correlation between the ND-O-BSA ELISA and the particle agglutination tests, the former measuring IgM antibodies to sugar terminal residues of PGL-I and the latter measuring antibodies to PGL-I as a whole, was in accordance with the previously reported high specificity for leprosy patients (2,3). We did not observe any increase in these immunoglobulins during ENL, as was reported by Cruickshank and Ellis and by Melsom, et al. (5,13).
Skin-test response to leprosin A has been reported to be negative in cases of lepromatous (LL,BL) leprosy (20). In our study, the response to leprosin A among those patients changed over time during treatment. There was a relation between skin-test responsiveness and the in vitro lymphoproliferative responses in MB patients during MDT. Those patients who had a high SI when exposed to antigen in vitro were positive with skin testing. There were, however, more patients who developed positive responses to M. leprae antigen in vitro by the end of 1 year of MDT than became positive in skin testing. This could be explained if a positive skin test at 72 hr is a local response to the soluble extrabacillary antigens of M. leprae (8). Such antigens would be only a part of the whole M. leprae sonicate antigen pool used to challenge the cells in the patient's blood in vitro. Serotaxonomic studies on M. leprae and M. vaccae have shown them to be similar in that both contain large amounts of common mycobacterial antigen(21). The changes observed in local skin responses to these antigens could be due to differences in responses to the group-specific antigens.
A few observations could be made on the cellular-immune response of patients undergoing reactions during treatment. Generally, responses decreased during ENL and increased during RR. Many studies have indicated that alterations occur in the cellular-immune responses during type 1 reactions, which often result in improvement within the clinical spectrum (10). Recent studies have also referred to a contribution made by cellular immunity to ENL (15), as opposed to earlier ideas which attributed a significant role to the presence of pathogenic immune complexes in ENL (18). In our study, the occurrence of reactions (ENL and RR) could be accompanied by changes in cellular responses. In RR, there was an increase in one case and a decrease in the other; the increase was in an upgrading RR. During an ENL episode of one patient, an increase then a decrease in cellular response could be seen. The results of this study would suggest that cellular-immune responses in vitro did not directly reflect any one state of reaction during treatment.
In conclusion, follow up of a series of leprosy patients during months to years of MDT confirmed or demonstrated the following: An exact relation between disease activity and IgM antibody levels was not clearly seen for MB patients; anti-M. leprae antibody levels fall with therapy at variable rates (1,4,7). Changes in in vitro cell-mediated immunity and in vivo skin-test responses seemed more directly related to the bacterial load, and might reflect the improvements of bacteriological and clinical parameters during MDT.
Acknowledgment. This work was supported by The Netherlands Ministry of Development cooperation through the VH-15 project between the University of Amsterdam, The Netherlands and the National Institute of Hygiene and Epidemiology, Hanoi, Vietnam. We are grateful to the Medicine Faculty of Hanoi for providing control blood. We would like to thank the World Health Organization and Dr. P. Klatser (Royal Tropical Institute, Amsterdam) for providing antigens and, again, Dr. Klatser for assistance in preparing the manuscript. We also would like to thank Dr. H. F. Engers (WHO) for very useful discussions and advice.
REFERENCES
1. Bach, M.-A., Wallach, D., Flageul, B., Hoffenbach, A. and Cottenot, F. Antibodies to phenolic glycolipid-I and to whole Mycobacterium leprae in leprosy patients: evolution during therapy. Int. J. Lepr. 54(1986)256-267.
2. Brett, S. J., Draper, P., Payne, S. N. and Rees, R. J. W. Serological activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52(1983)271-279.
3. Brett, S. J., Payne, S. N., Gigg, J., Burgess, P. and Gigg, R. Use of synthetic glycoconjugates containing the Mycobacterium leprae specific and immunodominant epitope of phenolic glycolipid 1 in the serology of leprosy. Clin. Exp. Immunol. 64(1986) 476-483.
4. Britton, W. J., Garsia, R. J. and Basten, A. Serological response to phenolic glycolipid of Mycobacterium leprae in Australian and Nepali leprosy patients. Aust. N. Z. J. Med. 17(1987)568-573.
5. Cruickshank, J. G. and Ellis, B. P. B. Leprosy and the serodiagnostic test for tuberculosis. J. Clin. Pathol. 30(1977)95-98.
6. Douglas, J. T., Naka, S. O. and Lee, J. W. Development of an ELISA for detection of antibody in leprosy. Int. J. Lepr. 52(1984)19-25.
7. Douglas, J. T., Steven, L. M., Fajardo, T., Cellona, R. V., Madarang, M. G., Abalos, R. M. and Steenbergen, G. J. The effects of chemotherapy on antibody levels in lepromatous patients. Lepr. Rev. 59(1988)127-135.
8. Fernandez, J. M. M. Estudio comparativo de la reacción de Mitsuda con las reacciones tuberculinicas. Rev. Argent. Dermatol. 23(1939)425-453.
9. Hussain, R., Jamil, S., Kifayet, A., Firdausi, F., Dockerell, H. M., Lucas, S. and Hasan, R. Quantitation of IgM Antibodies to the M. leprae synthetic disaccharide can predict early bacterial multiplication in leprosy. Int. J. Lepr. 58(1990)491-502.
10. Laal, S., Mishra, R. S. and Nath, I. Type I reactions in leprosy-heterogenicity in T-cell functions related to the background leprosy type. Int. J. Lepr. 55(1987)481-493.
11. Ly, H. M. and Stanford, J. L. Skin-testing of Vietnamese school-children with 15 new tuberculins. Tubercle 70(1989)27-36.
12. Lyons, N. F., Shannon, E. J., Ellis, B. P. B. and Naafs, B. Association of IgG and IgM antibodies to phenolic glycolipid-1 antigen of Mycobacterium leprae with disease parameters in multibacillary leprosy patients. Lepr. Rev. 59(1988)45-52.
13. Melsom, R., Harboe, M. and Naafs, B. Class specific anli-Mycobacterium leprae antibody assay in lepromatous leprosy (BL-LL) patients during the first two to four years of DDS treatment. Int. J. Lepr. 50(1982)271-281.
14. Melsom, R., Naafs, B., Harboe, M. and Closs, O. Antibody activity against Mycobacterium leprae antigen 7 during the first years of DDS treatment in lepromatous (BL-LL) leprosy. Lepr. Rev 49(1978)17-29.
15. Moudgil, K. D., Gupta, S. K., Naraynan, P. R., Srivastava, L. M., Mishra, R. S. and Talwar, G. P. Antibody response to phenolic glycolipid I and Mycobacterium w antigens and its relation to bacterial load in M. leprae -infected mice and leprosy patients. Clin. Exp. Immunol. 78(1989) 214-218.
16. Rao, T. D. and Rao, P. R. Enhanced cell-mediated immune response in erythema nodosum leprosum reactions of leprosy. Int. J. Lepr. 55(1987)36-41.
17. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
18. Roche, P. W., Britton, W. J., Failbus, S. S., Williams, D., Pradhan, H. M. and Theuvenet, W. J. Operational value of serological measurements in multibacillary leprosy patients: clinical and bacteriological correlates of antibody responses. Int. J. Lepr. 58(1990)480-490.
19. Sehgal, V. N. Reaction in leprosy; clinical aspects. Int. J. Dermatol. 26(1987)278-285.
20. Shield, M. J., Stanford, J. L., Garbajosa, G., Draper, P. and Rees, R. J. W. The epidemiological evaluation in Burma of the skin-test reagents, LRA6; a cell-free extract from armadillo derived Mycobacterium leprae. Part I: Leprosy patients. Int. J. Lepr. 50(1982)436-445.
21. Stanford, J. L. Skin testing with mycobacterial reagents in leprosy. Tubercle 65 (1984)63-74.
22. Tan Trao, V., Huong, P. L. T., Thuan, A. T., Long, H. T., Trach, D. D. and Wright, E. P. Responses to Mycobacterium leprae by lymphocytes from new and old leprosy patients: role of exogenous lymphokines. Ann. Inst. Pasteur/Immunol. 139 (1988) 121-133.
23. Truman, R. W., Shannon, E. J. and Hasting, R.C. Host responses to the phenolic glycolipid-1 antigen of M leprae. Int. J. Lepr. 53(1985)710-711.
24. Who Expert Committee On Leprosy. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
25. Who Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. M.Sc; Departments of Immunology and Bacteriology, National Institute of Hygiene and Epidemiology, Hanoi, Vietnam.
2. M.D.; Departments of Immunology and Bacteriology, National Institute of Hygiene and Epidemiology, Hanoi, Vietnam.
3. B.Sc; Departments of Immunology and Bacteriology, National Institute of Hygiene and Epidemiology, Hanoi, Vietnam.
4. M.Sc; Departments of Immunology and Bacteriology, National Institute of Hygiene and Epidemiology, Hanoi, Vietnam.
5. M.Sc; Departments of Immunology and Bacteriology, National Institute of Hygiene and Epidemiology, Hanoi, Vietnam.
6. B.Sc; Departments of Immunology and Bacteriology, National Institute of Hygiene and Epidemiology, Hanoi, Vietnam.
7. Ph.D., Departments of Immunology and Bacteriology, National Institute of Hygiene and Epidemiology, Hanoi, Vietnam.
8. H. Q. An, M.D.;National Institute for Dermatology, Hanoi, Vietnam.
9. M.D.; T. H. Khang, M.D.;National Institute for Dermatology, Hanoi, Vietnam.
10. M.D.;National Institute for Dermatology, Hanoi, Vietnam.
11. M.D., National Institute for Dermatology, Hanoi, Vietnam.
12. M.Sc; Department of Medical Microbiology, University of Amsterdam, Amsterdam, The Netherlands.
13. Ph.D., Department of Medical Microbiology, University of Amsterdam, Amsterdam, The Netherlands.
Reprint requests to Ms. Vu Tan Trao, % Dr. E. P. Wright whose present address is Royal Tropical Institute, Mauritskade 63, 1903 AD Amsterdam, The Netherlands.
Present address for Mr. Hendriks is National Institute of Health and Environmental Hygiene, Bilthoven, The Netherlands.
Received for publication on 14 July 1993.
Accepted for publication in revised form on 19 April 1994.