- Volume 62 , Number 3
- Page: 389–94
An eight-year field trial on antileprosy vaccines among high-risk household contacts in the Calcutta Metropolis
ABSTRACT
One-hundred-seventy-nine leprominnegative household contacts were vaccinated with heat-killed Mycobacterium leprae, BCG, or a combination of the two. Vaccination induced lepromin positivity in 131 of these contacts. Over an 8-year follow-up period, 12 lepromin-positive contacts developed leprosy, all tuberculoid; while 2 lepromin-negative vaccinated contacts developed leprosy, both lepromatous. Overall, 7.8% of the vaccinated contacts developed the disease.Seven-hundred-fourteen household contacts were not vaccinated, and served as controls. Among the 504 who were lepromin positive, leprosy developed in 35, all tuberculoid, over the 8-year follow up. Among the 210 lepromin-negative unvaccinated contacts, 61 developed leprosy: tuberculoid in 29, borderline in 4, lepromatous in 8, and indeterminate in 20. Overall, 13.5% of the 714 unvaccinated contacts and 29.0% of the 210 unvaccinated, leprominnegative contacts developed leprosy.
Vaccination could not induce lepromin positivity in all contacts. The three vaccines were equally effective in inducing lepromin positivity. Vaccination reduced the overall incidence of leprosy f rom 13.5% to 7.8% among household contacts but did not reduce the incidence of lepromatous leprosy (1.2% of all the vaccinated and 1.1% of all the unvaccinated contacts).
RÉSUMÉ
Cent septante-neuf contacts domiciliaires, négatifs à la lépromine, ont été vaccinés avec du Mycobacterium leprae tué par la chaleur, du BCG, ou une combinaison des deux. Le vaccin provoqua une positivation du test à la lépromine dans 131 de ces contacts. Au cours d'un suivi de 8 ans, 12 contacts positifs à la lépromine ont développé la lèpre, pour tous une forme tuberculoide; tandis que deux contacts vaccinés négatifs à la lépromine ont développé la lèpre, tous deux une forme lépromatcuse. Au total, 7,8% des contacts vaccinés ont développé la maladie.Sept cent quatorze contacts domiciliaires n'ont pas été vaccinés, et ont servi de témoins. Parmi les 504 qui étaient posiifs à la lépromine, la lèpre s'est développée chez 35, pour tous une forme tuberculoide, au cours du suivi de 8 ans. Parmi les 210 contacts non vaccinés négatifs à la lépromine, 61 ont développé la lèpre: tuberculoide dans 29 cas, borderline dans 4, lépromateuse dans 8 et indéterminée dans 20 cas. Au total, 13,5% des 714 contacts non vaccinés et parmi ceux-ci, 29,0% de ceux qui étaient négatifs à la lépromine, ont développé la lèpre.
La vaccination n'a pas pu provoquer une positivation du test à la lépromine chez tous les contacts. Les trois vaccins avaient la même efficacité pour provoquer une positivation du test. La vaccination a fait diminuer l'incidence globale de la lèpre de 13,5% à 7,8% parmi les contacts domiciliaires, mais n'a pas fait diminuer l'incidence de la lèpre lépromateuse (1,2% de tous les contacts vaccinés et 1,1 % de tous les contacts non vaccinés).
RESUMEN
Se vacunarom 179 contactos familiares leprominonegativos con Mycobacterium leprae muerto por calor, con BCG, o con una combinación de las dos micobacterias. La vacunación indujo positividad a la lcpromina en 131 de los contactos. En un período de seguimiento de 8 años, se encontró que 12 contactos lepromino-positivos desarrollaron lepra tuberculoide mientras que 2 contactos vacunados lepromino-negativos desarrollaron lepra lepromatosa. En total, 7.8% de los contactos vacunados desarrollaron la enfermedad.Setecientos catorce contactos familiares no fueron vacunados y sirvieron como controles. Entre los 504 que fueron lepromino-positivos, 35 desarrollaron lepra tuberculoide en un período de seguimiento de 8 años. De los 210 contactos no vacunados lepromino-negativos, 61 desarrollaron lepra: tuberculoide en 29, intermedia en 4, lepromatosa en 8, e indeterminada en 20. En conjunto, 13.5% del total de los contactos no vacunados y 29.0% de los contactos lepromino-negativos no vacunados, desarrollaron la enfermedad.
La vacunación no pudo inducir positividad a la lepromina en todos los contactos. Las 3 vacunas fueron igualmente efectivas como inductoras de positividad a la lepromina. La vacunación redujo la incidencia global de la lepra del 13.5 al 7.8% entre los contactos familiares, pero no redujo la incidencia de la lepra lepromatosa (1.2% de todos los contactos vacunados y 1.1% de todos los contactos no vacunados).
The aim of the present study was to protect lepromin-negative household contacts of leprosy patients against developing the disease by vaccination. A number of similar studies have been done in the past with BCG (1,10,13) vaccination, and different degrees of protection were achieved in different parts of the world. We used three vaccines, BCG, human Mycobacterium leprae, and a mixture of the two, in order to see if we could detect any differences among them in the degree of protection they offered. Recently Convit, et al. (5) have reported no differences in protection in household contacts in Venezuela between BCG and M. leprae and a combination of the two. We felt a study in India was appropriate since BCG alone does not seem to protect against leprosy in this country (2,9). There is evidence that BCG may even precipitate mild and transient forms of leprosy in those individuals with subclinical leprosy (14). To our knowledge, this is the first trial to be reported from India using these combination vaccines against leprosy.
MATERIALS AND METHODS
Human subjects
There were two groups of subjects (both household contacts of an active lepromatous or borderline leprosy patient), controls who received no vaccination, and vaccinated subjects. There were 714 healthy household contacts from 188 families who did not receive vaccination. Each was examined and showed no evidence of leprosy. They received lepromin skin tests; 504 (70%) were Mitsuda positive, the remaining 210 (30%) were negative. Another 179 household contacts from 96 families, all of whom were lepromin negative, were given leprosy vaccines.
Vaccines
Three different vaccines were used: a) heat-killed M. leprae of human origin, 1.6 x 107 in 0.1 ml; b) BCG (Japan), 1.5 x 105 in 0.1 ml; and c) a mixture of human M. leprae, 1.6 x 107 in 0.1 ml, and BCG (Japan), 1.5 x 10s in 0.1 ml.
Vaccine trial and follow up
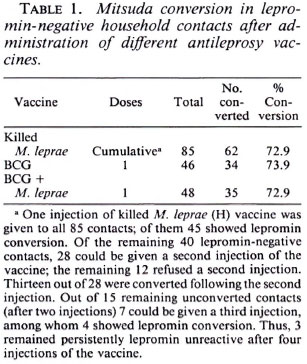
The 179 lepromin-negative household contacts were randomly divided into three groups: 85 received human M. leprae, 46 were given BCG, and 48 received the mixture of M. leprae and BCG. In the group receiving human M. leprae alone, 7 subjects were vaccinated three times at 6-month intervals, 28 twice at a 6-month interval, the remaining 50 subjects received only one vaccination. In the other two groups vaccines were administered only once (Table 1, footnote a).
All of the household contacts were skin tested with human lepromin, 1.6 x 106 in 0.1 ml. For those receiving vaccinations, lepromin skin testing was performed before and after vaccination.
All household contacts were followed for 8 years, beginning in 1985 at 6-month intervals. A diagnosis of leprosy was made on the basis of clinical examinations, slit-skin smears, and histopathological examinations of biopsies of any skin lesions which appeared.
Immunological testing
In those household contacts who were lepromin positive, either naturally or after vaccination, the following tests of specific cell-mediated immunity were performed:
Lepromin granuloma. The lepromin skintest site was biopsied and the granuloma studied histopathologically.
Capability of clearance of bacteria. This test was performed according to the method of Convit, et al. (4). Autoclaved human M. leprae (6.4 x 107 in 0.1 ml) were injected intradermally. Biopsies of the injected site were taken after 6 weeks and examined histopathologically.
Leukocyte migration inhibition (LMI) test. Leukocyte-rich plasma samples were obtained from the subjects; cell suspensions containing 4 x 106 cells per ml of minimal essential medium (MEM) were used to fill capillary tubes; these were incubated in migration chambers filled with MEM and 10% fetal calf serum with and without 1 x 107 M. leprae. Areas of migration and migration indexes were calculated (7).
RESULTS
Development of leprosy
Unvaccinated household contacts. During the 8 years of follow up, 96 (13.5%) of the 714 unvaccinated household contacts developed clinical leprosy. In 84 the disease was paucibacillary and in 12 it was multibacillary.
Of some interest, 504 of these 714 household contacts were lepromin positive; 35 (6.9%) of these 504 lepromin-positive contacts developed leprosy within 1 year of the lepromin testing, and all of them had tuberculoid disease. This might indicate that lepromin skin testing itself might precipitate overt tuberculoid disease in those with subclinical infections (14).
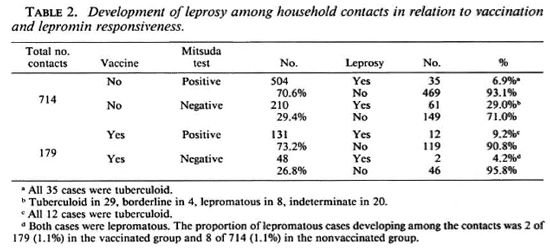
In the 210 lepromin-negative, unvaccinated contacts, 61 (29%) developed clinical leprosy. Of these 61 patients, 29 had tuberculoid leprosy, 4 borderline, 8 lepromatous and 20 indeterminate (Table 2).
Vaccinated household contacts. The lepromin conversion rates were similar after the three vaccine preparations. The cumulative lepromin conversions after up to three vaccinations with human M. leprae were 62 of 85 (73%); after a single vaccination with BCG, 34 of 46 (74%); after a single vaccination with the mixture of BCG and M. leprae, 35 of 48 (73%) (Table 1). Overall, 131 contacts became lepromin positive after vaccination and 48 remained lepromin negative.
Among the 131 contacts who developed a positive lepromin after vaccination, 12 (9.2%) developed leprosy during the 8-year follow up, all with tuberculoid disease (Table 2). Among the 48 contacts who remained lepromin negative after vaccination, 2 (4.1%) developed leprosy, both with lepromatous disease. Thus, overall 14 (7.8%) of 179 vaccinated, lepromin-negative contacts developed leprosy compared to 96 (13.5%) of all 714 unvaccinated contacts (p = 0.055, chi-squared, two-tailed, with Yates correction), and compared to 61 (29%) of 210 unvaccinated lepromin-negative household contacts (p < 0.00001, chisquared, two-tailed, with Yates correction).
Immunological testing. Immunological testing was possible in only 50 of 504 lcpromin-positive (spontaneous) contacts who did not receive any vaccination and in 126 of 131 vaccine-induced, lepromin-positive contacts.
Lepromin granuloma. All of the 176 granulomas examined histopathologically after lepromin skin testing were typical positive lepromin tests with Langhans' giant cells and lymphocytic infiltrations. There were no atypical features identified which were related to the development of tuberculoid leprosy.
Capability of clearance of bacteria. This test was negative in only two unvaccinated and eight vaccinated contacts. All 10 subjects showed a typical lepromin skin-test reaction histopathologically, and all 10 developed tuberculoid leprosy.
Leukocyte migration inhibition test. The LMI test was positive in all vaccinated contacts and, thus, had no relationship with the occurrence of disease.
DISCUSSION
Given leprosy's long incubation period and relatively low attack rates, a trial of a leprosy vaccine requires follow up of a relatively large number of people for a relatively long period of time. Restriction of the trial population to those with a relatively high attack rate, household contacts, allows some reduction in the sample size [WHO Report of the IMMLEP Subcommittee meeting on the planning of leprosy vaccine trials. TDR/IMMLEP(subtrial)/80.3 (1980) pp. 1-36].
Trial description
In the present study we have tested the efficacy of three potential antileprosy vaccines for 8 years in 179 vaccinated subjects compared to 714 unvaccinated subjects, all of whom were household contacts of active borderline or lepromatous cases.
Lepromin conversion About 71% of household contacts are (natural) lepromin positive. In the present study about 73% of initially lepromin-negative household contacts could be lepromin converted after administration of one to four doses of vaccine, while 27% remained lepromin-negative despite the fact that they were immunized.
Vaccination and development of leprosy
The total number of leprosy cases among 714 unvaccinated contacts was 46 (13.5%) which was brought down to 7.8% (14/179) by vaccination (Table 2). This difference perhaps was due to vaccine-induced lepromin conversion. Lepromin positivity was no guarantee that an individual would not get leprosy, however. In fact, 35 (6.9%) of 504 lepromin-positive individuals, who were not vaccinated, developed leprosy and 12 (9.2%) of 131 vaccinated lepromin-positive contacts developed leprosy. What is of considerable interest is that all 47 of 635 (7.4%) lepromin-positive contacts (unvaccinated and vaccinated) developed tuberculoid leprosy. In contrast, among the 210 unvaccinated lepromin-negative contacts the 61 (29%) cases who developed leprosy had disease across the whole leprosy spectrum, from tuberculoid to lepromatous, and only the 2 (4.2%) of 48 of vaccinated but lepromin-negative contacts had leprosy of only lepromatous type. Thus, 63 (24.4%) of 258 lepromin-unresponsive (unvaccinated and vaccinated) contacts developed leprosy, which was much higher than the incidence of leprosy (7.9%) among lepromin-positive contacts. Vaccination certainly offered protection to the contacts against leprosy by making them lepromin positive.
When the incidence of leprosy cases among the unvaccinated lepromin-negative and vaccinated lepromin-negative contacts were compared, it was found that the rates of the development of lepromatous leprosy were identical in both groups, i.e., 1.1% (8/ 714) and 1.1% (2/179), respectively. This indicated that vaccination could not reduce the rate of the development of multibacillary lepromatous leprosy among the household contacts. However, since there was no occurrence of unstable borderline leprosy among the vaccinated contacts, it might be speculated that perhaps vaccination shifted those contacts at risk of developing borderline leprosy, if they remained unvaccinated, toward the tuberculoid end. This notion was supported by the increased incidence (9.2%) of tuberculoid leprosy among contacts, who had undergone lepromin conversion following vaccination, in comparison to the low incidence of tuberculoid leprosy (6.9%) in 540 unvaccinated, but lepromin-reactive (natural) contracts (Table 2).
BCG vaccination and its limitations
BCG alone and BCG plus killed M. leprae vaccines could effectively induce lepromin conversion in some contacts (Table 1). But BCG vaccination was often associated with severe local reactions at injection sites and lymphadenitis in those who had BCG vaccination in their childhood. These reactions were absent, however, in contacts who were given combined vaccines, perhaps due to the immunosuppressive action of M. leprae and their components, PGL-I and LAM (6,12). From two different recent studies the efficacy of BCG vaccination in childhood for the prevention of leprosy appeared to be limited: One from India showed development of indeterminate leprosy in 3.7% of the children of parents with leprosy who received BCG vaccination (11); the other from Pakistan showed that BCG vaccination could not reduce the occurrence of lepromatous leprosy (8). Furthermore, we found that BCG-induced lepromin responsiveness is weak and not permanent, while spontaneous lepromin reactivity (natural) was intense and life-long. This was because BCG vaccination in lepromin-negative contacts could perhaps activate macrophages nonspecifically and failed to offer M. leprae specific T-cell function.
Assessment of immunologic augmentation offered by vaccination
Surrogate immunologic markers of protective immunity against leprosy not being available, the following in vivo tests were employed: a) traditional lepromin skin test with killed M. leprae (human origin), b) study of histological types (typical or atypical) of lepromin-induced granulomas, c) ability of clearance of bacteria from granuloma induced by injection of killed M. leprae, and d) leukocyte migration inhibition test (LMI), an in vitro test.
The lepromin test showed that the grades of Mitsuda positivity were variable and age dependent. In lepromin-reactive (natural) contacts the degrees of lepromin positivity were 1+ in the age group of 5 to 10 years, 2 + in 11 -20 years, and 3 + in contacts older than 20 years. On the other hand, among vaccine-induced, Mitsuda-positive contacts, 1 + positivity was observed in the age group of 5 to 10 years, and 2+ positivity was found in those older than 10 years of age. Thus, the grades of lepromin reaction were more intense among spontaneously lepromin-positive contacts than those in vaccine-induced, lepromin-positive subjects. Furthermore, in contrast to the former group the lepromin positivity tended to fade away 1 to 2 years after vaccination in the latter groups. Despite this weak and transient vaccine-induced lepromin reactivity, only 12 of 131 contacts who underwent lepromin conversion following vaccination developed tuberculoid leprosy, and none of the rest presented with disseminated illness during the 8-year study period (Table 2). The occurrence of tuberculoid leprosy in 47 out of 635 lepromin-positive (natural and vaccine-induced) contacts (Table 2) showed that lepromin positivity could not be taken as a hallmark for protection against tuberculoid leprosy. Nevertheless, Table 2 shows that lepromin-positive (natural and vaccine-induced) contacts did not develop lepromatous leprosy and, thus, it might be taken as an indicator of resistance against disseminated disease.
All of the 47 vaccine-induced, leprominpositive contacts who developed tuberculoid leprosy showed typical delayed-type hypersensitivity granuloma after lepromin challenge and positive LMI tests against challenge with M. leprae sonicate, but 10 of them failed to clear acid-fast bacilli from the lepromin-induced granuloma. This perhaps could explain why some leprominpositive contacts developed tuberculoid leprosy.
It is postulated that protection against leprosy has two components: a) intact M. leprae- driven , specific T-lymphocyte function which, in turn, activates b) macrophages loaded with M. leprae and eliminates them. It is a matter of despair that although vaccination could evoke specific T-lymphocyte function in the contacts, as evidenced by the positive LMI test, and could stimulate macrophage function, as shown by lepromin-induced delayed-type hypersensitivity granuloma (typical) formation, it failed to show a positive CCB test in 10 contacts who developed tuberculoid leprosy. Is this defect (CCB negativity) associated with macrophage dysfunction which could not be corrected by vaccination? This needs further exploration.
On the other hand, development of lepromatous leprosy in two contacts, who remained lepromin unreactive following vaccination, needs no explanation. These contacts perhaps had failure of M. leprae specific T-lymphocyte function (3). Unfortunately, at the present time we do not possess any vaccine that could regenerate lost M. leprae -specific T-cell clone function in those contacts who are persistently lepromin unreactive even after multiple vaccination. These contacts are at high risk of developing lepromatous leprosy.
Acknowledgment. The authors are grateful to the Directorate of Science and Technology, New Delhi, for a financial grant to the senior author.
REFERENCES
1. Bechelli, L. M., Lwin, K., Gallego-Garbajosa, P., Gyi, M. M., Vemora, K. and Sundaresan, T. BCG vaccination of children against leprosy: nineycar findings of the controlled WHO trial in Burma. Bull. WHO Org. 51(1974)93-99.
2. Bloom, B. R. and Jacob, W. R. New strategics for leprosy and tuberculosis and development of bacillus Calmette-Guerin into a multivaccine vehicle. Ann. N.Y. Acad. Sci. 569(1989)155-173.
3. Britton, W. J. Leprosy 1962-1992; immunology of leprosy. Trans. R. Soc. Trop. Med. Hyg. 87(1993)508-514.
4. Convit, J., Avila, J. L., Goihman, M. and Pinardi, M. E. A test for the determination of competency in clearing bacilli in leprosy patients. Bull. WHO 46(1972)821-826.
5. Convit, J., Sampson, C, Zuniga, M., Smith, P. G, Plata, J., Silva, J., Molina, J., Pinardi, M. E., Bloom, B. R. and Salgade, A. Immunoprophylactic trial with combined Mycobacterium leprae /BCG vaccine against leprosy: preliminary results. Lancet 339(1992)446-450.
6. Fournie, J. J., Adams, E., Mullins, R. J. and Beston, A. Inhibition of human lympho-proliferative responses by mycobacterial phenolic glycolipids. Infect. Immun. 57(1989)3653-3659.
7. Godal, T., Myrvang, B., Stanford, J. L. and Samuel, R. Recent advances in the immunology of leprosy with special references to new approaches in immunoprophylaxis. Bull. Inst. Pasteur 72(1974)273-310.
8. Mohyuddin, G. BCG vaccination and leprosy. Indian J. Lepr. 64(1992)545.
9. Muliyil, J., Nelson, K. E. and Diamond, E. L. Effect of BCG on the risk of leprosy in an endemic area: a case control study. Int. J. Lepr. 59(1991)229-236.
10. Russel, D. A. BCG vaccination in the prophylaxis of leprosy: the Karimui Leprosy Research Group. (Abstract) Int. J. Lepr. 41(1973)617.
11. Saha, K., Rao, K. N., Chattopadhya, D., Lakshmi, V., Gadi, S. and Datta Banik, N. D. A study of nutrition, growth and development of a high risk group of children of urban leprosy patients. Eur. J. Clin. Nutr. 44(1990)471-480.
12. Shepard, C. C, Walker, L. L., Van Landingham, R. and Ye, S.-Z. Sensitization or tolerance to Mycobacterium lepra antigen by route of injection. Infect. Immun. 38 673-680.
13. Stanley, S. J., Howland, C, Stones, M. M. and Sutherland , I. BCG vaccination against leprosy in Uganda: final results. J. Hyg. (Camb.) 87 (1981) 233-248.
14. Stoner, G. L., Belehu, A., Nsibambi, J. and Warndorff, J. Borderline tuberculoid leprosy following BCG vaccination; a case report. Int. J. Lepr. 49 16-20.
1. M.B.B.S., D.C.P., Ph.D., Professor of Leprology (deceased), School of Tropical Medicine, Calcutta 700073, India.
2. M.B.B.S., D.T.M.&H., D.C.P., Ph.D., Professor of Leprology, School of Tropical Medicine, Calcutta 700073, India.
3. M.B.B.S., M.D., Demonstrator in Leprology, School of Tropical Medicine, Calcutta 700073, India.
4. M.B.B.S., Demonstrator in Leprology, School of Tropical Medicine, Calcutta 700073, India.
5. M.B.B.S., School of Tropical Medicine, Calcutta 700073, India.
6. M.B.B.S., M.D., Assistant Director of Microbiology, National Institute of Communicable Diseases, 22 Sham Nath Marg, Delhi 110054, India.
7. M.Sc, M.B.B.S., Ph.D., Professor of Immunology (retired), Vallabh Bhai Patel Chest Institute, Delhi University, Delhi 110007, India.
Reprint requests to Dr. Saha, 45A Seva Bazar Street, Calcutta 700005, India.
Received for publication on 12 April 1993.
Accepted for publication in revised form on 16 March 1994.