- Volume 62 , Number 3
- Page: 395–8
Transmission of leprosy in nude mice through thorn pricks
ABSTRACT
The dorsum of the feet of 10 nude mice was smeared with 107 Mycobacterium leprae and then pricked with cactus thorns contaminated with M. leprae. In 15 months five of them developed lepromatous nodules at the infected site and disseminated lesions in the ears, nose, tail and the organs of the reticuloendothelial system. Penetrating injuries through unprotected skin contaminated with M. leprae f rom the environment may play a role in the transmission of leprosy in humans.RÉSUMÉ
On a maculé la face dorsale des pattes de 10 souris nues avec 107 Mycobacterium leprae, et on l'a ensuite piquée avec des épines de cactus contaminées par M. leprae. En quinze mois, cinq d'entre elles ont développé des nodules lépromateux au site d'infection et des lésions disséminées dans les oreilles, le nez, la queue et les organes du système réticulo-endothélial. Des blessures pénétrant à travers une peau non protégée contaminée avec des M. leprae de l'cnvironncmencnt pourraient jouer un rôle dans la transmission de la lèpre aux êtres humains.RESUMEN
El dorso de las patas traseras de 10 ratones desnudos se impregnó con 107 Mycobacterium leprae y después se arañó con espinas de cactus contaminadas con M. leprae. En 15 meses, 5 de los ratones desarrollaron nodulos lepromatosos en los sitios infectados y lesiones diseminadas en las orejas, la nariz, la cola y los órganos del sistema reticuloendotclial. Las heridas penetrantes en la piel no protegida contaminada con M. leprae del ambiente pueden jugar un papel en la transmisión de la lepra en los humanos.The route and mode of transmission of leprosy is a subject of continued interest. In our earlier studies using nude mice, we have demonstrated that Mycobacterium leprae was transmitted through unbroken nasal mucosa (1), abraded skin of the cooler parts of the body (4), and by subcutaneous and intravenous injections (2). Nine-banded armadillo leprosy in the wild is well documented (7) and a large number of them carry thorns embedded in their ears, noses and foot pads (6). It has been shown that there is a distinct possibility of these animals getting infected with M. leprae through thorn pricks (6). In this study we report our attempts to infect nude mice through thorn pricks.
MATERIALS AND METHODS
A group of 10 nude mice was used in this study. The animals were anesthetized by intraperitoneal injections of sodium pentobarbital at 60 mg/kg body weight. The anesthetized animals were placed in the prone position and 10 µl of M. leprae suspension containing 1 x 109 bacilli per ml (1 x 107 bacilli) were placed on the dorsal surface of both hind feet. The bacilli were from the foot pad of a nude mouse inoculated 10 months earlier with M. leprae. Harvesting and preparation of the bacillary suspension was as previously described (2).
The thorn pricks were made using bunny ear cactus (Opuntia microdasys). This cactus has clusters of uniform and fine spines and, therefore, injury to the skin would be minimal and the depth of the thorn pricks could be controlled. A young ear of the cactus plant was removed and sterilized by exposure to formaldehyde vapor for 18 hr. One cluster of thorns was removed using a 5-mm punch, was dipped in the same mycobacterial suspension, and was pressed against the dorsum of the mouse foot where the M. leprae suspension was previously placed. The cactus tissue was then withdrawn. It was found that many of the thorns were broken and remained embedded in the skin of the animal. The bacillary suspension applied to the skin was allowed to dry, and the animal was returned to a recovery cage and observed until it regained consciousness. The mice were then maintained in a pathogen-free environment and observed until they were sacrificed and autopsied 15 and 17 months later.
Tissues removed during autopsy for histopathologic studies were fixed in 10% buffered formalin and processed for paraffin sections. Sections at 4-µm thickness were made and stained with hematoxylin and eo-sin (H&E) and a modified Fite's stain for acid-fast bacilli (AFB).
RESULTS
Five of the 10 experimental mice survived. They developed a minimal but noticeable swelling of the dorsum of the hind feet in 6 months which gradually increased in size and appeared as large nodules at 15 months. These nodules resembled the lesions observed in nude mice injected subcutaneously with M. leprae. One animal was sacrificed at 15 months and another at 17 months, and both were autopsied. The hind and fore feet, ears, nose, tail, liver, spleen, lungs, heart, kidneys, adrenal glands and organs of the gastrointestinal system were studied histopathologically. The other three mice died at 17 months due to a power failure following a hurricane, and only the hind foot pads of these animals could be recovered for histopathologic study.
Hind feet
Sections through the hind feet showed a granuloma occupying largely the dorsum of the foot (Fig. 1). The granuloma consisted of foamy macrophages with vacuolated cytoplasm and a few diffusely scattered lymphocytes. Small clumps of neutrophils were also present here and there. The granuloma surrounded and infiltrated striated muscles, tendons, hair follicles, nerves and bones, partly destroying and replacing them in some areas. The epidermis was stretched and thinned out with a clear zone separating it from the granuloma. Acid-fast staining showed vacuoles of the macrophages packed with AFB (Fig. 2). AFB also were present in striated muscles, perineurial cells and Schwann cells.
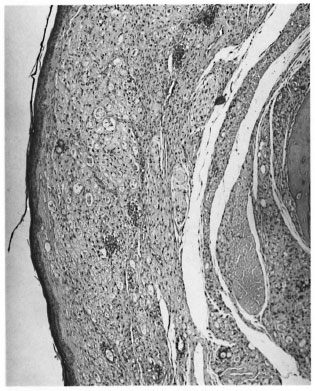
Fig. 1. Photomicrograph of the nodule on the dorsum of a nude mouse foot pad composed mostly ofsubepithelial collections of macrophages (H&E x 60).
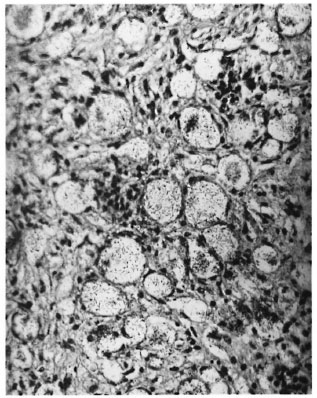
Fig. 2. High-power view of Figure 1, showing large vacuoles in macrophages containing M. leprae (mod-ified Fite's stain x 750).
Ears
Sections through both ears showed small nodular collections of macrophages and a few scattered lymphocytes placed on either side of the cartilage (Fig. 3). The granuloma surrounded the cartilage, nerves, fatty tissue and striated muscle. AFB were present in large numbers in macrophages, nerves and striated muscles.
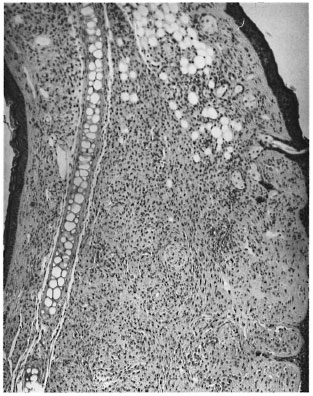
Fig. 3. Photomicrograph of a nodule from the ear showing collections of macrophages on either side ofthe cartilage (H&E x 60).
Nose
Sections of the nose showed many foamy macrophages infiltrating the striated muscle and mucous glands. Acid-fast staining showed bacilli inside muscle cells and macrophages.
Tail
Sections through the tail showed small focal collections of macrophages which on acid-fast staining had many AFB.
Liver, spleen and lungs
Sections showed a few diffusely scattered reticuloendothelial cells in the lungs, spleen, and liver containing many AFB. A few small macrophage collections containing AFB also were present in the portal areas of the liver.
Other organs
None of the other organs examined, such as the heart, kidneys, adrenal glands and organs of the gastrointestinal system, showed any lesions.
DISCUSSION
In this experimental study fine cactus thorns were used to produce minute penetrating injuries to the mouse skin and subcutaneous tissue which did not produce any obvious bleeding. The injured skin and the thorns were contaminated with M. leprae. The results of the study showed beyond any doubt that there was entry of adequate numbers of viable M. leprae through the thorn pricks to produce a lepromatous nodule in 15 months. While there was no way of assessing the exact number of viable M. leprae that gained entry into the skin, the rate of development of swelling was similar to that seen in nude mice that received 1 x 104 organisms by routine injection into the plantar surfaces of the hind foot pad (2). If approximately 1 x 104 organisms did reach the deeper areas from the 1 x 107 bacilli applied to the skin surface plus those adhering to the thorns, then approximately 1 in 1000 surface M. leprae were inoculated by the thorn pricks.
It is well known that viable M. leprae can exist in the environment up to 46 days (3). In an endemic area, a large number of bacilli are shed into the environment from multibacillary patients and perhaps from yet unidentified sources. In countries where a large number of people walk barefoot and many children are not fully clothed, the chances of these organisms adhering to the surface of exposed skin are great. Further, in these parts of the world the exposed skin is often liable to injury. Ulcers of the skin due to scabies and other superficial skin infections are common. Injuries that would drive M. leprae into the skin may be an important mechanism for the transmission of leprosy in humans.
The time taken for the appearance of a lesion following the introduction of M. leprae is long even in nude mice. If a similar mechanism is involved in humans it would be almost impossible to associate a minor skin injury with the eventual appearance of a leprosy lesion. Further, in earlier studies using armadillos, a lesion may not always appear at the site of introduction of M. leprae. The smaller the number of organisms inoculated, the lesser the chances of seeing a lesion at the site of entry of the bacilli (5). Protective clothing and footwear to reduce skin injuries may help control leprosy in developing countries where the disease is endemic.
REFERENCES
1. Chehl, S., Job, C. K. and Hastings, R. C. Transmission of leprosy in nude mice. Am. J. Trop. Med. Hyg. 34(1985)1161-1166.
2. Chehl, S., Ruby, J., JOB, C. K. and HASTINGS, R. C. The growth of M. leprae in nude mice. Lepr. Rev. 54(1983)283-304.
3. Desikan, K. V. and Sreevastava. Studies on viability of M. leprae outside the human body. Lepr. India 513(1979)588-589.
4. Job, C. K., Chehl S. and Hastings, R. C. New findings on the mode of entry of M. leprae in nude mice. (Letter) Int. J. Lepr. 58(1990)726-729.
5. Job, C. K., Drain, V., Truman, R., Deming, A. T, Sanchez, R. M. and Hastings, R. C. The pathogenesis of leprosy in the nine-banded armadillo and the significance of IgM antibodies to PGL 1. Indian J. Lepr. 64(1992)137-152.
6. Job, C. K., Harris, E. B., Allen, J. L. and Hastings, R. C. Thorns in armadillo car and noses and their role in the transmission of leprosy. Arch. Pathol. Lab. Med. 110(1986)1025-1028.
7. Walsh, G. P., Storrs, L. E., Burchfield, H. P., Cottrell, E. H., Vidrine, M. F. and Binford, C. H. Leprosy-like disease occurring naturally in armadillo. J. Rcticulocndothel. Soc. 18(1975)347-351.
1. C. K. Job, M.D., F.R.C. Path.;Laboratory Research Branch, GWL Hansen's Disease Center at Louisiana State University, P.O. Box 25072, Baton Rouge, Louisiana 70894, U.S.A.
2. S. K. Chehl, M.S.;Laboratory Research Branch, GWL Hansen's Disease Center at Louisiana State University, P.O. Box 25072, Baton Rouge, Louisiana 70894, U.S.A.
3. R. C. Hastings, M.D., Ph.D., Chief, Laboratory Research Branch, GWL Hansen's Disease Center at Louisiana State University, P.O. Box 25072, Baton Rouge, Louisiana 70894, U.S.A.
Reprint requests to Dr. Job at his present address: St. Thomas Hospital and Leprosy Center, Chettupattu 606801, T.S. District, Tamil Nadu, India.
Received for publication on 7 December 1993.
Accepted for publication in revised form on June 14, 1994.
Yo Yuasa, M.D., kindly served as Acting Editor in regard to the submission, review, revision and acceptance of this manuscript.