- Volume 62 , Number 3
- Page: 353–8
Relapse rates in patients treated with dapsone monotherapy and combinations of dapsone and thiambutosine, thiacetazone, isoniazid and streptomycin in the pre-MDT era
ABSTRACT
Relapse rates were studied in patients f rom northern Thailand who were started on dapsone monotherapy between 1949 and 1976. Included are a group of patients who, for various reasons, also received combinations of dapsone and thiambutosine, thiacetazone, isoniazid and streptomycin. The overall relapse rate in paucibacillary patients on dapsone monotherapy only was 2.7 per 1000 person-years at risk (PYR) (average observation period 13.9 years). In the multibacillary patients who received dapsone monotherapy only, the relapse rate was 10.5 per 1000 PYR (average observation period 12.4 years). In both groups it was found that 50% of the relapses occurred after the seventh year of follow up. The overall relapse rate in those patients whose treatment included thiambutosine, thiacetazone, isoniazid and/or streptomycin for at least 3 months was 17.9 per 1000 PYR (average observation period 11.9 years). The difference with the multibacillary patients treated with dapsone monotherapy only is not significant. It is concluded that alternative antileprosy drugs included in therapy regimens with dapsone in the pre-MDT era did not result in relapses occurring less often.RÉSUMÉ
Les taux de récidive ont été étudiés chez des malades du nord de la Thailande qui avaient commencé une monothérapie à la dapsone entre 1949 et 1976. On y a inclu un groupe de malades qui, pour différentes raisons, avaient aussi reçu des combinaisons de dapsone et thiambutozine, thiacetazone, isoniazide et streptomycine. Le taux global de récidive des malades paucibacillaires mis sous la seule monothérapic à la dapsone était de 2,7 pour 1000 personnes-années à risque (PAR) (période moyenne d'observation 13,9 ans). Chez les malades multibacillaires sous la seule monothérapie à la dapsone, le taux de récidive était de 10,5 pour 1000 PAR (période moyenne d'observation 12,4 ans). On a observé dans les deux groupes que 50% des récidives survenaient après la septième année de suivi. Le taux global de récidive chez les malades dont le traitement comprenait de la thiambutosine, du thiacetazone, de l'isoniazide et/ou de la streptomycine pour un minimum de 3 mois était de 17,9 pour 1000 PAR (période moyenne d'observation 11,9 ans). La différence avec les malades multibacillaires traités seulement par dapsone en monothérapie n'est pas significative. On en conclut que les médicaments anti-lèpre alternatifs inclus avec la dapsone dans les régimes thérapeutiques qui ont précédé l'époque de la PCT n'ont pas résulté en une diminution des récidives.RESUMEN
Se estudió la frecuencia de recaídas en pacientes del norte de Tailandia que empezaron su tratamiento de monoterapia con dapsona entre 1949 y 1976. Así mismo se incluyó un grupo de pacientes que, por diversas razones, también recibieron combinaciones de dapsona y tiambutosina, tiacetasona, isoniazida y estreptomicina. Se encontró que, en un período promedio de observación de 13.9 años, la frecuencia general de recaída en los pacientes paucibacilares tratados sólo con dapsona fue de 2.7 por 1000 personas-años en riesgo (PYR). En los pacientes multibacilares que recibieron sólo la monoterapia con dapsona la frecuencia de recaída fue de 10.5 por 1000 PYR en un período promedio de observación de 12.4 años. En ambos grupos se encontró que 50% de las recaídas ocurrieron después del séptimo año de seguimiento. La frecuencia global de recaída en aquellos pacientes tratados además con tiambutosina, tiacetasona, isoniazida y/o estrepromicina, cuando menos durante 3 meses, fue de 17.9 por 1000 PYR en un período promedio de observación de 11.9 años. La diferencia con los pacientes multibacilares tratados sólo con dapsona no fue significativa. Se concluye que las drogas antileprosas alternativas incluidas en el esquema de tratamiento con dapsona antes de la era de la poliquimioterapia no disminuyó la frecuencia de recaídas.Dapsone (DDS) was introduced as antileprosy treatment at the end of the 1940s. It was generally very successful, and DDS monotherapy remained the mainstay of leprosy treatment until 1982 when the World Health Organization (WHO) advised introducing multidrug therapy (MDT) (9). Over the years several problems had arisen with dapsone. In the first place it was found that certain people could not tolerate the drug, usually because of hypersensitivity to sulfones. Also, resistance of the leprosy bacteria to DDS became more frequent; many cases relapsed, often many years after completing treatment. These problems necessitated the search for and introduction of alternative antileprosy drugs. Eventually this led to the recommendation by the WHO to use MDT. In MDT, dapsone is combined with rifampin in paucibacillary (PB) cases, and with rifampin and clofazimine in multibacillary (MB) cases.
In order to assess the efficiency of the presently used treatment schedules, it is important to have insight into the results of past treatment schedules. In this paper the results of three decades of dapsone monotherapy are analyzed, with reference to relapse rates in both PB and MB patients. Also, a similar analysis will be done for combination therapies given before the MDT era. Combinations that were reviewed include dapsone with the following drugs: thiambutosine, thiacetazone, isoniazid and streptomycin.
MATERIALS AND METHODS
All leprosy patients included in this study were treated at McKean Rehabilitation Centre or associated clinics. The center is located in Chiang Mai, northern Thailand. It is a church-related institution, serving leprosy sufferers since 1908. Until 1970 there was a large colony with approximately 1000 inpatients. Apart from the colony residents, many more people were treated as outpatients. Most patients originated from northern Thailand, but some came from other parts of the country.
Dapsone monotherapy was started in 1949. MDT, as advised by the WHO, was introduced in 1982. Between 1957 and 1982 a number of alternative antileprosy drugs were used including Rimifon (INAH), thiacetazone (TBI), thiambutosine (CIBA 1906) and streptomycin. These drugs were usually introduced when patients were considered "intolerant" to dapsone. In practice, this meant that the patient was undergoing a leprosy reaction, usually type 2 (erythema nodosum leprosum, ENL). Dapsone was interrupted and the alternative drug was given for a period of time. Often dapsone was resumed at a later stage. Thus usually sequential (not combined), multidrug therapy was received by a number of the patients. It is appreciated that sequential monotherapy does not allow synergistic activity between drugs, but on many occasions there was overlapping of drugs for 1 to 3 months, especially when DDS was being re-introduced, and isoniazid and streptomycin were usually given together. The DDS dosage regimens used since 1949 at McKean Rehabilitation Centre have been published elsewhere (1).
All patients who started dapsone between were treated with dapsone monotherapy only and patients who were treated with dapsone combined with at least one of the above-mentioned drugs given for at least 3 months were included. In the analysis of monotherapy, both PB and MB cases were included. In the analysis of combined therapy, only MB cases were taken into consideration.
Patients selected for analysis needed to have completed at least 3 years of dapsone monotherapy if they belonged to the PB group (TT and BT with negative skin smear at presentation). Patients belonging to the MB group (BT with positive skin smear at presentation, BB, BL and LL) needed to have taken dapsone only or dapsone and the alternative drug until they became skin-smear negative (defined as having a negative skin smear on three consecutive examinations with at least a 3-month interval). These periods are referred to as the "treatment" period.
After the treatment period, an "observation" period commenced for the calculation of person-years at risk (PYR). During this period most patients continued dapsone monotherapy according to the then existing guidelines. To differentiate in the degree of therapy compliance, a distinction was made between regular users (taking >70% of prescribed therapy) and irregular users (taking <70% of prescribed therapy). The observation period was discontinued when: the patient died; was lost for follow up; started MDT; relapsed or reached the end of the study period (1991).
Relapse was defined as: a) the reappearance of a positive bacterial index (seen twice with at least a 6-month interval) after at least 3 years of skin-smear negativity, combined with new clinical activity in skin or nerves; and b) the reappearance of new, clinical lesions (even with skin-smear negativity) after an adequate course of therapy.
The relapse rate was calculated by dividing the total number of relapses by the total of person-years of observation (PYR).
RESULTS
Of the 2296 patients treated with dapsone monotherapy, 1147 belonged to the PB group and 1149 to the MB group.
Table 1 shows the figures for the PB group. A total of 43 patients relapsed during an accumulated observation period of 15,969 years (PYR), giving an overall relapse rate of 2.7 per 1000 PYR. The relapse rate among patients who took dapsone regularly was 1.7 per 1000 PYR; among the irregular users this figure was 3.6 per 1000 PYR, the difference being significant (p < 0.05). Relapse rates in males were 2.4 (regular) and 4.2 (irregular) per 1000 PYR. In females these rates were 0.4 (regular) and 2.5 (irregular) per 1000 PYR. The difference between regular males (2.4) and regular females (0.4) is significant (p < 0.05). The relapse rate between irregular males and females is not significant.
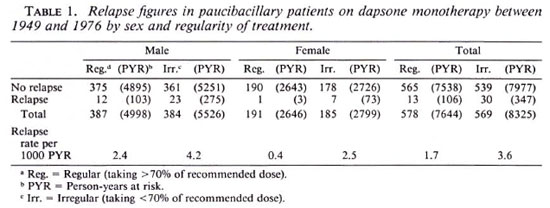
Table 2 summarizes the results for MB patients treated with dapsone monotherapy. In this group there were 150 cases with relapse observed during an accumulated period of 14,297 years, giving an overall relapse rate of 10.5 per 1000 PYR. The relapse rate among patients who took dapsone regularly was 9.9 per 1000 PYR; among the irregular users this figure was 10.8 per 1000 PYR. Relapse rates in males were 9.8 (regular) and 12.5 (irregular) per 1000 PYR. In females these rates were 10.2 (regular) and 6.4 (irregular) per 1000 PYR. There were no significant differences between males and females and no significant differences between regular and irregular patients.
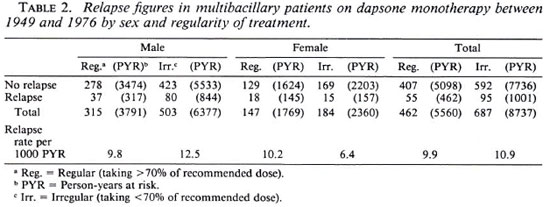
Table 3 shows the results found in MB patients who had been treated with dapsone together with an alternative drug. The alternative drug was taken for an average duration of 1.5 years (3 months to 15 years). The number of relapses observed in this group is 34, seen during an accumulated period of 1904 years. The overall relapse rate is 17.9 per 1000 PYR. The difference in relapse rate in regular users (15.0) and irregular users (19.6) is not significant (p > 0.05). The difference between regular males (13.8) and irregular males (22.1) is significant (p < 0.05). The difference between regular (17.9) and irregular (12.8) females is not significant. There are no significant differences between regular males and females and no significant differences between irregular males and females.
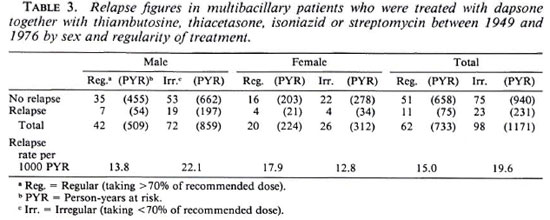
Comparing the PB and MB groups of patients who had received dapsone monotherapy, there is a highly significant difference between the two groups. Between the groups of MB patients who had received either monotherapy or combined/sequential therapy, the difference in the overall relapse rate per 1000 PYR and between the regular users is not significant. The difference between the irregular users is significant, the combination therapy group showing a higher relapse rate.
Table 4 gives the leprosy classification as originally seen at the time the patient first presentated and at the time when the relapse occurred.
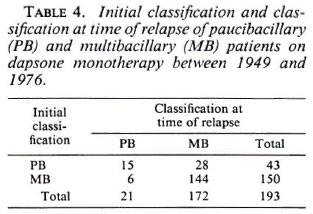
The Figure shows the percentage of relapse per year of observation for the PB and MB groups. It is noted that in both groups 50% relapsed after the seventh year of observation.
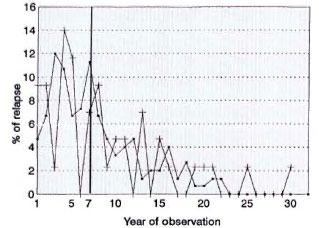
The figure. Percent relapses per year of observation. -■- = Multibacillary; -+- = paucibacillary.
DISCUSSION
The main features of this study, compared to most previous studies, are the long period of observation after completing the treatment period and the inclusion of the group of patients who had received a combination of antileprosy drugs in the pre-MDT era. The main difference with many similar studies is the fact that the group of patients included in this study continued to use dapsone during their observation period.
In the PB group, the overall relapse rate over an average observation period of 13.9 years (1-30 years) was 2.7 per 1000 PYR (3.7% of the total group). Jesudasan, et al. observed a relapse rate in 1701 PB patients during a 3-year period of follow up of 9.7 per 1000 PYR (4). A relapse rate of 7.2 per 1000 PYR over an average period of 6.6 years was found by Becx-Bleumink (1). Pandian, et al. found an overall relapse rate of 5 per 1000 PYR, followed up to a maximum of 15 years (6). The finding in the present study of a relapse rate of 2.7 per 1000 PYR (1.7 per 1000 PYR in regular patients) is significantly lower than the above-mentioned studies. Most likely this is due to the fact that these patients continued dapsone monotherapy during the observation period. The figure is comparable with that found for relapse rates in PB patients after completing MDT (2). Half of the observed relapses in this PB group occurred in the first 7 years of observation. At the time of relapse, 65% were reclassified as MB.
The average period of observation in the MB group on dapsone monotherapy only was 12.4 years (1-22 years). The overall relapse rate was 10.5 per 1000 PYR (13% of the total group). This overall rate is not significantly different (p > 0.05) from that found in comparable studies by Kurz, et al. (5) and Cartel, et al. (3) of MB patients who also continued dapsone during the observation period.
The first study, among Indian patients, showed an overall relapse rate of 12.8 per 1000 PYR. In the second study, conducted in Polynesia, a relapse rate of 13.9 per 1000 PYR was found. The overall relapse rate is also comparable with the study by Waters, et al. (8), who described relapses in 362 institutionalized lepromatous patients. These patients had been treated for about 20 years with fully supervised dapsone before the drug was discontinued. They were actively followed up for 8-9 years, and a relapse rate was found of 10.4 per 1000 PYR. More recently Becx-Bleumink reported a relapse rate among MB patients of 24.8 per 1000 PYR after being released from dapsone monotherapy on an average of 6.6 years previously (1). The same author reported a relapse rate of 2.4 per 1000 PYR for MB patients after completing MDT with an average follow up of 4.7 years (2).
As with the PB group, only half of the relapses in the present study occurred in the first 7 years of observation. The MB patients who received dapsone together with an alternative antileprosy drug had an average follow up of 11.9 years. The overall relapse rate was 17.9 per 1000 PYR (21% of the total group). The difference with the MB group of patients who received dapsone monotherapy only is not significant (p > 0.05). From this study it is shown that the inclusion of thiambutosine, thiacetazone, isoniazid or streptomycin for at least 3 months in the therapy regimen has not been of any benefit at all in terms of the relapse rate.
In conclusion, it can be noted that the findings of this study basically confirm the conclusions reached in previous reports as far as relapse rates expected in PB and MB patients on dapsone monotherapy. It illustrates the superiority of the currently used MDT regimens, both from an operational and economical point of view. It also emphasizes the need that all MB patients who have been treated with dapsone, regardless the duration, should receive MDT. However, this study does show that half of the relapses in the PB and MB groups described occur after 7 years of follow up. This should teach us that, even with MDT, relapses can be expected after many years, and provision should be made for this when planning on policies for the follow up of patients released from treatment after MDT. Since long-term follow up is not feasible in most programs, patients who have completed MDT should be educated about the need to report back immediately if any new signs or symptoms of active disease develop. The inclusion of thiambutosine, thiacetazone, isoniazid and/ or streptomycin for at least 3 months in therapy regimens with dapsone in the pre-MDT era did not prevent relapses from occurring.
REFERENCES
1. Becx-Bleumink, M. Relapses in leprosy patients after release from treatment from dapsone monotherapy; experiences in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Int. J. Lepr. 60(1992)161-172.
2. Becx-Bleumink, M. Relapses among leprosy patients treated with multidrug therapy: experience in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia; practical difficulties with diagnosing relapses; operational procedures and criteria for diagnosing relapses. Int. J. Lepr. 60(1992)421-435.
3. Cartel, J.-L., Boutin, J. P., Spiegel, A., Plichart, R. and Roux, J. F. Longitudinal study on relapses of leprosy in Polynesian multibacillary patients on dapsone monotherapy between 1946 and 1970. Lepr. Rev. 62(1991)186-192.
4. Jesudasan, K., Christian, M. and Bradley, D. Relapse rates among nonlepromatous patients released from control. Int. J. Lepr. 52(1983)304-310.
5. Kurz, X. M., Declerq, E. E. and Vellut, C. M. Rate and time distribution of relapses in multibacillary leprosy. Int. J. Lepr. 57(1989)599-606.
6. Pandian, T. D., Muliyil, J. and Vellut, C. Risk of relapse among non-lepromatous patients released from treatment after dapsone monotherapy. Lepr. Rev. 62(1991)288-296.
7. Richardus, J. H. and Smith, T. C. Increased incidence of hypersensitivity reactions to dapsone after introduction of multidrug therapy. Lepr. Rev. 60(1989)267-273.
8. Waters, M. F. R., Rees, F. J. W., Laing, A. B. G., Khoo Kah Fah, Meade, T. W., Parikshak, N. and North, W. R. S. The rate of relapse in lepromatous leprosy following completion of twenty years of supervised sulphone therapy. Lepr. Rev. 57(1986)101-109.
9. Who Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. M.B.B.S.; McKean Rehabilitation Center, P.O. Box 53, Chiang Mai 50000, Thailand.
2. M.D., Ph.D.; McKean Rehabilitation Center, P.O. Box 53, Chiang Mai 50000, Thailand.
Received for publication on 22 December 1993; accepted for publication in revised form on 28 April 1994.