- Volume 62 , Number 3
- Page: 428–32
Arthritis in leprosy: clinical, laboratory, and radiological assessments
In an effort to increase the utility of the Journal in continuing medical education, in this section we welcome contributions dealing with practical problems in leprosy work. Submissions to this section will undergo minimal editorial changes and may well contain controversial points. Letters to the Editor pointing out other viewpoints are welcome.
Leprosy is a chronic infectious disease caused by Mycobacterium leprae. Considerable attention has been devoted to the dermal, neural and osseous complications of leprosy.1 Reports of joint involvement in leprosy have been published since the 1960s.2-8 However, only a few studies have highlighted the clinical pattern of joint involvement in leprosy.2,3 The present study was undertaken with a view to delineate the clinical pattern of arthritis in leprosy, to study the serological and radiological changes, and to follow the course of arthritis for a period after starting the treatment of leprosy. The main aspect of interest was to study the joint involvement in leprosy patients not in reaction since the arthritis in lepra reaction is well known.5-8
MATERIALS AND METHODS
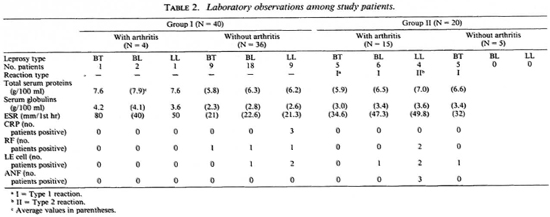
Forty untreated leprosy patients (Group I) and 20 untreated leprosy patients in lepra reaction (Group II) participated in the study from among the patients attending the Leprosy Clinic of the Department of Dermatology, Venereology and Leprology attached to the Nehru Hospital, Postgraduate Institute of Medical Education and Research, Chandigarh, India. Out of the 40 patients in Group I, 27 were males and 13 were females with a mean (S.E.M.) age of 31.4(1.24) years and a mean duration of disease of 2.18 (0.27) years. Out of the 20 patients in Group II, 13 were males and 7 were females with a mean (S.E.M.) age of 30 (2.47) years and a mean duration of disease of 1.3 (0.14) years. The diagnosis of leprosy was confirmed in each case from skin smears and skin-biopsy specimens and classified according to the method of Ridley and Jopling9(Table 1). In Group II, 16 patients (10 BT and 6 BL) were in type 1 reaction; 4 patients (all LL) were in type 2 reaction (Table 2). Clinical and laboratory criteria including dermal histopathology were used to diagnose type 1 and type 2 reactions.1
A detailed history was taken of peripheral articular disease, particular attention being paid to a personal or family history of any of the co-diseases of seronegative spondar thritides.10 Any history of joint stiffness or pain was taken. The pattern of development of articular symptoms was noted, and in all patients the joint tenderness score was obtained on the Ritchie articular index.11 Hemoglobin concentration, white cell count, erythrocyte sedimentation rate (ESR), and total and differential serum proteins were measured at the initial examination. Lupus erythematosus (LE) cell preparation, antinuclear factor (ANF), latex fixation test for rheumatoid factor (RF), and C-reactive protein (CRP) were done as part of the serology. Standard radiographs of the hands, feet and knees were assessed by one radiologist who did not know the clinical or laboratory results. The above laboratory investigations and radiological assessment were done for all the patients of both groups irrespective of the presence of arthritis clinically.
The patients were started on antileprosy treatment as per the World Health Organization multidrug therapy (WHO/MDT) recommendations,12 and the course of arthritis was assessed clinically at 3 months, 6 months and 1 year after starting the treatment.
Nonparametric tests were used for the statistical analysis of the data.
RESULTS
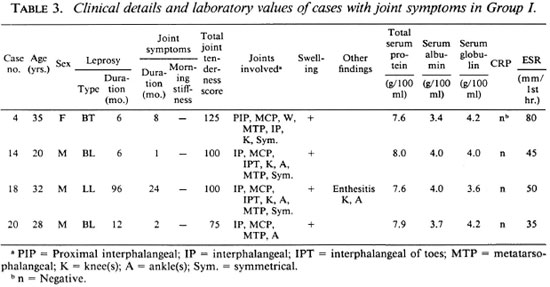
Four (10%) of the 40 patients in Group I had evidence of arthritis clinically. Fifteen (75%) of the 20 patients in Group II had evidence of arthritis clinically (Table 2). The association of Group II with arthritis was significant (p < 0.001). In Group I, out of four patients who had arthritis the duration of leprosy exceeded the duration of arthritis in three patients (Table 3). None of the patients in either group gave a history of morning stiffness or gelling (stiffness after inactivity). The mean joint tenderness score in Group I and Group II was 100 and 141.33, respectively. In both groups the interphalangeal joints, metatarsophalangeal joints, wrists, ankles and knees were involved in the patients having arthritis. The arthritis was bilaterally symmetrical although the tenderness score varied at times in the same joint on two sides of the body. All the affected joints showed mild-to-moderate swelling, but there was no evidence of gross effusion or synovial thickening in any of the affected joints. None of the joints was marginally hotter than the surrounding normal skin. No crepitus was felt on movement of the joints although a degree of stiffness was evident on passive movements. Motion was limited in all directions and planes of movement. Both active and passive movements were limited by pain. None of the patients had obvious deformity of the joints. Periarthritis (enthesitis) was noted, affecting the knees and ankles of one patient with arthritis in Group I (Table 3) and two patients in Group II. None of the patients had any subcutaneous or tendinous nodules in relation to the affected joints or elsewhere.
The observations on total serum proteins, serum globulins, ESR, CRP, and RF are shown in Table 2. The ESR values and the serum globulins were higher in Group I patients having arthritis compared to those without arthritis; however, these higher values were comparable with those of Group II patients with or without arthritis (Table 2).
CRP was positive in three LL patients without arthritis in Group I (Table 2); in all other patients in Groups I and II it was negative. RF was negative in all four patients having arthritis in Group I. It was negative in all patients without arthritis in this group except for three patients (Table 2). RF was positive in two patients having arthritis in Group II. The results of the ANF and LE cell testing are shown in Table 2. ANF was positive in three LL patients having arthritis in Group II; two of these patients had a positive LE cell test.
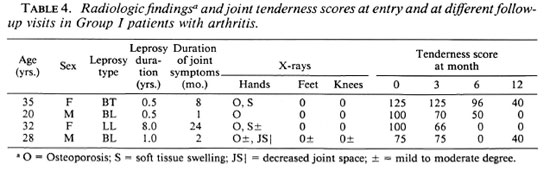
Periarticular osteoporosis was the most common radiological finding affecting leprosy patients having arthritis in Group I (Table 3) and Group II. It involved the small joints of hands and feet, wrist joints, ankle joints and knee joints in a symmetrical fashion, and varied in degree from moderate to severe. At times, osteoporosis around the affected joints was more than what could be expected from disuse. There was a reduction in the transverse trabeculae in the subarticular layer and a diminution of the longitudinal layers of trabeculae in the cortex. In the small bones of the hands, irregularity of the medullary side of the cortex was observed with areas of patchy erosion in the shaft. However, cortical expansion was not observed. The other changes observed were soft tissue swelling and a diminution of joint space. No evidence of calcification, subperiosteal new bone formation, bone cyst formation or honeycombing was observed. A correlation between the degree of loss of bone density and clinical joint involvement was observed. Radiological observations in Group I and Group II patients not having arthritis essentially were normal.
Patients in Group I were started on antileprosy treatment, and it was noticed that their joint symptoms started improving with a decrease in pain and swelling. Range of movement and grip strength improved in the small joints of the hands. Objectively, the joint tenderness scores fell from initial values to zero in two patients at the end of 1 year (Table 4). None of these patients went into reversal reaction. The patients in Group II also were given steroidal or nonsteroidal antiinflammatory agents as needed in addition to antileprosy treatment. None of these patients had joint symptoms at the end of 1 year.
DISCUSSION
The symmetrical polyarthritis observed in this study differs in a number of ways from arthritis in leprosy recorded previously. 2-8 Firstly, both peripheral and proximal joint involvement was found. Secondly, the arthritis was symmetrical. Thirdly, the arthritis did not differ in clinical presentation and evolution within any leprosy subgroup. Fourthly, the arthritis was nonerosive. It responded symptomatically to antileprosy treatment.
The preliminary nature of our study meant that the incidence and prevalence of arthritis in patients with leprosy could not be established because of the small number of patient samples. The study highlights, however, that a symmetrical polyarthritis of the synovial joints may be a facet of the leprosy infection. The study also shows that arthritis in leprosy per se or as a component of lepra reaction has some similarity to rheumatoid arthritis with regard to certain of its clinical features. Although the pattern of distribution of joint pains was somewhat like early rheumatoid arthritis, concomitant rheumatoid arthritis is unlikely because of the lack of clinical or radiological evidence of underlying destructive arthritis, the absence of rheumatoid factors, the lack of extra-articular manifestations of rheumatoid arthritis, and the response of the arthritis to antileprosy therapy. The evolution of the arthritis and the pattern of joint involvement, in the absence of systemic disturbances, do not correspond to any arthritis reported previously in leprosy patients in India.
The higher mean tenderness score of Group II patients having arthritis as compared to that of Group I patients having arthritis shows that the degree of tenderness and functional disability was higher in the former as compared to the later.
Acute and chronic arthritis associated with erythema nodosum leprosum (ENL) reactions may represent an Arthus reaction or a reactive arthropathy, and is restricted to the lepromatous end of the leprosy spectrum.13 In contrast, the arthritis we report here in Group I (leprosy patients without reaction) was of gradual onset, encompassed the whole leprosy spectrum and had no other clinical features of ENL. We postulate that inflammatory arthropathy in leprosy patients without reaction may be an important factor in joint destruction in the later stages of disease in addition to the wellknown neurogenic arthropathy, and may be due to an underlying immune complex disease. The elevation of ESR and serum globulins in these patients can be explained on the basis of stimulation of the immune response by chronic infection with M. leprae resulting in hypergammaglobulinemia and production of autoantibodies.14,15 The nonspecific indicator of chronic inflammation (raised ESR) seems to correlate well with the elevation of serum globulins in these patients (Table 2). Serology including rheumatoid factor, antinuclear factor and LE cell preparations was negative so the arthritis was seronegative.
The presence of arthritis was positively associated with periarticular osteoporosis. Other workers have also reported joint osteoporosis as the most common radiological abnormality.2,8 No radiological evidence of articular erosions was observed by us. However, erosive arthritis in leprosy has been reported.2 The relatively short duration of leprosy could be the reason for the absence of radiological erosions in our patients having arthritis.
Further studies with larger numbers of patients are warranted to establish the incidence and prevalence of arthritis in nonreactional leprosy. A larger series of patients is also required to confirm the negative serological results.
- Inderpal Singh, M.B.B.S.
Resident
- Surrinder Kaur, M.D., F.A.M.S.
Professor and Head
Department of Dermatology, Venereology and Leprology
- Niranjan Khandelwal, M.D.
Associate Professor
Department of Radiodiagnosis
- Inderjeet Kaur, M.D.
Associate Professor
Department of Dermatology, Venereology and Leprology
- Shridhar D. Deodhar, M.D., M.A.M.S.
Professor
Department of Internal Medicine
Postgraduate Institute of Medical Education & Research
Chandigarh 160012, India
1. Jopling, W. H. and McDougall, A. C. The disease. In: Handbook of Leprosy. 4th edn. London: Heinemann, 1988, p. 10; 86.
2. Atkin, S. L., El-Ghobarey, A., Kamel, M., Owen, J. P. and Dick, W. C. Clinical and laboratory studies of arthritis in leprosy. Br. Med. J. 298(1989)1423- 1425.
3. Atkin, S. L, Welbury, R. R., Stanfield, E., Beavis, D., Iwais, B. and Dick, W. C. Clinical and laboratory' studies of inflammatory polyarthritis in leprosy patients in Papua New Guinea. Ann. Rheum. Dis. 46(1987)688-690.
4. Alcocer, J. V., Herrera, C. L., Gudino, J. and Fraga, A. Inflammatory arthropathy in leprosy. (Abstract) Arthritis Rheum. 22(1979)587.
5. Karat, A. B. A., Karat, S., Job, C. K. and Furness, M. A. Acute exudative arthritis in leprosy: rheumatoid arthritis like syndrome in association with erythema nodosum leprosum. Br. Med. J. 3(1966)770-773.
6. Lele, R. C, Sainani, G. S. and Sharma, K. D. Leprosy presenting as rheumatoid arthritis. J. Assoc. Physicians India 13(1965)275-277.
7. Modi, T. H. and Lele, R. D. Acute joint manifestations in leprosy. J. Assoc. Physicians India 17(1969)247-254.
8. Ramu, G. and Balakrishnan, S. Arthritis in lepromatous leprosy-clinical features and biochemical findings. Lepr. India 40(1968)62-69.
9. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
10. Wright, V. Relationship between ankylosing spondylitis and other spondarthritides. In: Ankylosing Spondylitis. Moll, J. M. A., ed. London: Churchill Livingstone, 1980, p. 42.
11. Ritchie, D. M., Boyle, J. A. and Mclnnes, J. M. Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. Q. J. Med. 37(1968)393-406.
12. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech Rep. Ser. 657.
13. Sharma, V. K., Saha, K. and Sehgal, V. N. Serum immunoglobulins and autoantibodies during and after erythema nodosum leprosum. Int. J. Lepr. 50(1982)159-163.
14. Bonomo, L., Pinto, L., Dammacco, F. and Barbieri, G. Thyroglobulin antibodies in leprosy. Lancet 2(1963)807-809.
15. Bonomo, L., Tursi, A., Trimigliozzi, G. and Dammacco, F. LE cells and antinuclear factors in leprosy. Br. Med. J. 2(1965)689-690.
Reprints requests to Dr. Deodhar.