- Volume 62 , Number 3
- Page: 444–5
Status of HBV DNA and HBsAg in leprosy patients
To the Editor:
Hepatitis due to "B" infection and mycobacterial disease are still major problems of the developing world and a possible association between hepatitis B virus (HBV) infection and leprosy has been proposed (1,4). The data available to date arc based on HBsAg status alone and are inconclusive, mainly due to the lack of consistency in the methods used for detection (6,7). In the present study we investigated the correlation of HBV infection with different types of leprosy where, in addition to HBsAg, cloned HBV DNA was used as a marker of ongoing HBV infection.
Forty-one patients belonging to different types of the spectrum of leprosy, classified clinically and histologically according to Ridley-Jopling (8), were used in the study. HBV DNA analysis was done by a dot blot assay which had a detection limit of 3 x 104 virus particles (0.1 pg DNA) from 200 µ l of patient's serum (2). HBsAg was assayed by Abbot EIA using a commercial kit according to manufacturer's instructions. The results are summarized in The Table. It is evident that the incidence of HBV infection is more in LL leprosy, suggesting a correlation between the HBV infection and the cell-mediated immune response to Mycobacterium leprae. Almost 50% of the patients in the LL category were found to have cither HBV DNA or HBsAg in their scrum (The Table, E). However, detailed analyses of individual cases indicated that the presence of HBsAg or HBV DNA alone is not sufficient to draw any conclusions about the status of HBV infection. Out of 41 samples analyzed, only 3 were found to be positive to both HBV DNA and HBsAg. In the LL category, five cases were picked up by the DNA probe although they were negative for HBsAg. This was not surprising, and could be due to a higher sensitivity offered by molecular hybridization assays (2).
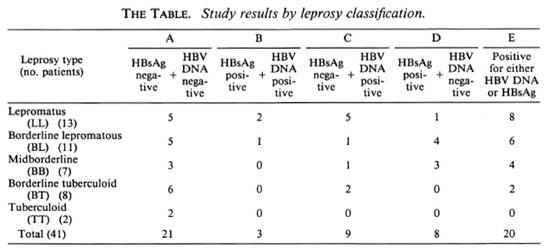
On the other hand, 4 of 13 BL and 3 of 7 BB leprosy patients did not show any detectable HBV DNA in their sera, although they were HBsAg positive. The presence of HBsAg in serum in the absence of HBV DNA has been reported when HBV DNA becomes integrated into hepatocellular chromosomes (5). A similar situation also exists in the case of acute viral hepatitis where HBsAg appears in the serum before HBV DNA. In the high cell-mediated immune response category (BT, TT) only 2 of 10 patients showed HBV DNA; all were negative for surface antigen. The HBsAg carrier rate in India is reported to be 4%-6%. In a control study, out of 150 HbsAg-negative, apparently healthy individuals on the basis of clinico-biochemical criteria, 9 were found to be HBV DNA positive (2).
Taken together, HBV infection in healthy individuals by HBsAg/HBV DNA criteria is not more than 10%.
The available information on the prevalence of HBV infection in leprosy patients is based on HBsAg detection alone, but without any conclusion. This is due to varying degrees of sensitivity of HBsAg detection (1,7) offered by various techniques used by different investigators, such as CIE, RIA, RPHA, ID, ELISA. Different mechanisms proposed by various investigators include genetic susceptibility to HBV infection, impaired cell-mediated immunity in LL patients, increased risk of exposure to virus due to institutionalization or increased exposure to virus due to its high incidence in the surrounding population. Involvement of genetic factors in acquiring HBV infection also was proposed by Blumberg and his colleagues (3). Contrary to this, Fakunlc and Whittle (6) reported that the exposure rate to leprosy patients was similar to that of a control population, suggesting that these particular leprosy patients did not have any predisposition to HBV infection.
Although the present studies were carried out with a limited number of patients, the observation strongly suggest a high prevalence of HBV infection (about 50%) compared to the controls which had a HBsAg carrier rate between 4%-6% (2). This observed high prevalence of HBV infection is more evident in the BL and LI categories (60%) when compared with the BT and TT categories (20%) and suggests a relationship between decreased cell-mediated immunity and HBV infection. The results further stress the need for using a DNA probe based upon a molecular hybridization assay in addition to HBsAg detection in order to draw a meaningful conclusion.
- Kakoli Banerjee, Ph.D.
Gene Expression Laboratory
- Saurabhi Ghosh, Ph.D.
Hybridoma Laboratory
National Institute of Immunology
New Delhi 110067, India
- K. D. Moudgil, Ph.D.
Department of Biochemistry
All India Institute of Medical Sciences
New Delhi 110029, India
- Pramod Khandekar, Ph.D.
Gene Expression Laboratory
National Institute of Immunology
New Delhi 110067, India
Acknowledgment. The authors wish to thank Dr. Subrata Sinha, Department of Biochemistry, AIIMS, New Delhi, and Dr. Satyajit Rath, National Institute of Immunology, for their suggestions and constructive criticism. Thanks also go to Mr. Sunder Bisht for typing the manuscript. The work was supported by grants to the National Institute of Immunology from the Department of Biotechnology, Government of India.
REFERENCES
1. Balkrishnan, S., Bhatia, V. N. and Hari-Krishnan, S. Hepatitis B surface antigen in leprosy patients. Lepr. India 55(1983)45-48.
2. Banerjee, K., Sharma, G., Upadhyay, S., Anand, Raju, G. S. and Khandekar, P. S. Detection of hepatitis B virus in blood samples negative for surface antigen by DNA probe hybridization assay. J. Biosci. 14(1989)279-287.
3. Blumberg, B. S. and Melartin, L. Lepromaous leprosy and Australia antigen with comments ongenetics of leprosy. J. Chron. Dis. 23(1970)507-516.
4. Blumberg, B. S., Melartin, L., Lechat, M. and Guinto, R. S. Association between lepromatousleprosy and Australia antigen. Lancet 2(1967)173-176.
5. Brechot, C., Degos, F., Lugassy, C., Thiers, V., Zafrani, S., Franco, D., Bismuth, H., Trepo, C., Benhamon, J., Wands, J., Issenlbacher, K., Tiollais, P. and Berthlot, P. Hepatitis B virus DNA DNA in patients with chronic liver disease and negative tests for hepatitis B surface antigen. N. Engl. J. Med. 312(1985)270-274.
6. Fakunle, Y. M. and Whittle, H. C. Hepatitis B virus infection in patients with leprosy: a serologicalstudy in a leprosarium in northern Nigeria. Trans.R. Soc. Trop. Med. Hyg. 75(1981)623-625.
7. .MOUDGIL, K. D. and IRSHAD, M. Global overview of the prevalence of hepatitis B virus markers (HBsAg and anti-HBS) in leprosy patients. Trop.Gastroenterol. 9(1988)184-190.
8. RIDLEY, D. S. and JOPLING, W . H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
Reprint requests to Dr. Khandekar.