- Volume 62 , Number 2
- Page: 237–44
Detection and characterization of a λgt 11 recombinant clone of M. leprae that expresses an antigenic determinant of a 64-kDa protein
ABSTRACT
A genomic library of Mycobacterium leprae in the expression vector λgt 11 was screened with rabbit polyclonal hyperimmune antiserum elicited with a sonicated extract of M. leprae. Numerous reactive clones were isolated by this immunoscrcening, indicating a broad antibody response of the rabbit. None of the recombinant clones was reactive with monoclonal antibodies to the previously well-characterized M. leprae recombinant clones. One of the clones isolated, clone A, encoded a large, 132-143-kDa β-galactosidase fusion protein expressing an epitope of M. leprae. Monospecific antibodies eluted f rom this fusion protein reacted on Western blots with a 64-kDa M. leprae protein. The DNA of this clone was shown to be distinet f rom the gene encoding the well-characterized immunodominant 65-kDa protein by DNA hybridization. We have identified a new λgt 1 1 recombinant clone encoding a fusion protein with a 64-kDa protein. This protein is recognized by the humoral immune response of the rabbit in response to a challenge with an M. leprae cell extract.RÉSUMÉ
Une librairie génomique de Mycobacterium leprae dans le vecteur d'expression λgt 1 1 a été testée avec de l'antisérum polyclonal hyperimmun de lapin sensibilisé avec un extrait soniqué de M. leprae. De nombreux clones réactifs ont été isolés par ce dépistage immunologique, indiquant une réponse étendue du lapin sous forme d'anticorps. Aucun des clones recombinants ne réagissait avec les anticorps monclonaux dirigés vers les clones recombinants de M. leprae bien caractérisés antérieurement. Un des clones isolés, le clone A, encodait une grande protéine de fusion β-galactosidase de 132-143 kDa exprimant un epitope de M. leprae. Des anticorps monospécifiques produits à partir de cette protéine de fusion réagissaient par Western blot avec une protéine de 64 kDa de M. leprae. On a montré que l'ADN de ce clone était distinct du gène encodant la protéine immunodominante de 65 kDa bien caractérisée par hybridation de l'ADN. Nous avons identifié un nouveau clone recombinant λgt 1 1 encodant une protéine de fusion avec une protéine de 64 kDa. Cette protéine est reconnue par la réponse immunitaire humorale du lapin en réponse au contact avec un extrait cellulaire de M. leprae.RESUMEN
Se analizó una genoteca de Mycobacterium leprae en el vector de expresión lambda-gt1 1, usando un antisucro hiperinmune policlonal, preparado en conejo contra un extracto sonicado de M. leprae. Se aislaron numerosas clonas reactivas que indicaron una amplia respuesta del conejo. Sin embargo, ninguna de las clonas recombinantes reaccionó con los anticuerpos monoclonales contra las clonas recombinantes de M. leprae previamente bien caracterizadas. Una de las clonas aisladas, la clona A, codificó para una proteína de fusión con beta-galactosidasa de 132-143 kDa que expresó un epitopo de M. leprae Los anticuerpos monocspecíficos cluídos de la proteína de fusión reaccionaron en Western blots con una proteína de 64 kDa de M. leprae. Por análisis de hibridización, el DNA de esta clona fue distinto del gene codificante de la ya bien caracterizada proteina inmunodominante de 65 kDa. Identificamos una nueva clona recombinante lambda-gt 1 1 codifeante de una proteína de fusión con una proteína de 64 kDa. Esta proteína es reconocida por la respuesta inmune humoral del conejo en respuesta a su inmunización con un extracto celular de M. leprae.Awareness of the disease leprosy dates to biblical times yet the causative agent, Mycobacterium leprae, is one of the last remaining infectious disease pathogens to be fully characterized antigenically. The evasiveness of M. leprae results from both our inability to culture the mycobacteria for prolonged periods of time and its limited host range. When organisms are recovered from infected tissues, sonicated, and subjected to polyacrylamide gel electrophoresis there are many proteins visible after staining (14,26) Relatively few of these 30 plus proteins have been identified and characterized (12); thus the nature of most proteins has yet to be defined. More information about the M. leprae proteins is important for vaccine development and studies of the pathogenesis of the organism.
In 1985, Young, et al. prepared a M. leprae genomic expression library in λgt 11 that they screened for expressed proteins with mouse monoclonal antibodies to M. leprae (33). Their studies and those of other researchers (2,24) led to the isolation of genes encoding defined antigens of M. leprae. The most frequently studied proteins, 12kDa, 18kDa, 28kDa, 36kDa, 65kDa, and 70kDa, expressed in Escherichia coli, have been a reliable source of antigens to study humoral and cell-mediated immune responses in leprosy patients. Several of these proteins are homologous with heat-shock proteins (22,29), and the 28-kDa, 65-kDa, and 70-kDa antigens are present in at least three other mycobacterial species (2,3,30). Additional genes have been identified by screening a plasmid expression library of M. leprae (11), by complementation studies in auxotrophic bacteria (8, 15), and by the immunoscreening of the λgt 1 1 expression library with sera from leprosy patients (6, 17. 21, 27).
In an effort to identify additional mycobacterial antigens we screened the λgt 1 1 M. leprae recombinant library of Young, et al. (33) with hyperimmune polyclonal rabbit antiserum elicited with a sonicated extract of M. leprae, with the hypothesis that the rabbit antibody response following M. leprae immunization might differ significantly from the antibody repertoire of mice such as that indicated by monoclonal antibodies (9,10). A similar approach to screening a λgt 1 1 M. tuberculosis genomic library was undertaken by Young, et al. (31) Species variability in the antibody response to M. leprae has been shown by the studies of Vadiee, et al. (26). They showed that M. leprae infected nine-banded armadillos, an important animal model in the study of leprosy, generally recognized and produced antibodies to a greater diversity and larger number of M. leprae antigens than did multibacillary leprosy patients. We report a description of a previously uncharacterized recombinant clone that encodes a β-galactosidase fusion protein expressing an epitope of a 64-kDa M, leprae protein.
MATERIALS AND METHODS
Bacteria, λgt 1 1 clones, and antisera. E. coli strains Y1089 and Y1090 have been described previously (32). These strains were obtained from Drs. T. P. Gillis (Laboratory Research Branch, GWL Hansen's Disease Center at Louisiana State University, Baton Rouge, Louisiana, U.S.A.) and L. L. Muldrow (Clark Atlanta University, Atlanta, Georgia, U.S.A.), respectively. The genomic expression library of M. leprae in λgt 1 1 and the recombinant lambda clones Y3180 and Y3179, expressing the 36-kDa and 18 kDa genes (33), respectively, were kindly provided by Dr. R. A. Young (Whitehead Institute, Cambridge, Massachusetts, U.S.A.). Plasmid pUC3 and λgt 1 1 clone Y3178, both expressing the 65-kDa gene of M. leprae, and monoclonal antibodies IVD8, IIC8, IIH9, and E42β were provided by Dr. Gillis. Monoclonals SA1D2D1, Yl-2, F479, 46.7, L5, and ML06-A1 were provided through the IMMLEP Program of the World Health Organization. Rabbit hyperimmune antiserum to M. leprae cell sonicates was supplied by Dr. R. Navalkar (Morehouse School of Medicine, Atlanta, Georgia, U.S.A.).
Immunological screening of the λgt 1 1 M. leprae genomic library. Bacteriophages from the M. leprae λgt 1 1 expression library were plaqued in Luria-Bertani (LB) top agar on lawns of E. coli Y1090 at 42ºC. The plaques were induced simultaneously and transferred 4 hr later at 37ºC onto nitrocellulose filters (Bio-Rad Laboratories, Richmond, California, U.S.A.) prctreated with 10 mM isopropyl-β-D-thiogalactopyranoside (IPTG; Promega, Madison, Wisconsin, U.S.A.). They were subsequently screened with hyperimmune polyclonal rabbit antiserum. The immunoscreening procedure was essentially that described by Huynh, et al. (13), except that extraneous protein-binding sites were blocked with 5% nonfat dry milk in TBS (Tris buffered saline, 50 mM Tris-HCl, pH 8.0, 150 mM NaCl) and the reactive clones were detected with the ProtoBlot alkaline phosphatase (AP) system (Promega). The rabbit antiserum waspreabsorbed with E. coli lysate and used at a 1:100 dilution. Plaque-purified clones were also probed with monoclonal antibodies at a 1:200 dilution. AP-conjugated antibodies (goat anti-rabbit and goat anti-mouse) were used at a 1:7500 dilution.
Construction and induction of lysogens. Lysogens of clone A, Y3178, and Y3179 were constructed in E. coli Y1089 as described C3). An E. coli lysogen of clone Y3I80 was obtained from Dr. T. N. Shinnick (Centers for Disease Control, Atlanta, Georgia, U.S.A.). The growth and induction of lysogens were according to standard procedures (13) as described previously (16). Cells were pelleted, resuspended in 1% sodium dodecyl sulfate (SDS), and stored at -80ºC.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) of lambda lysogens. Induced lambda E. coli lysogens were resolved on 10% polyacrylamide slab gels using the discontinuous buffer system of Lacmmli (l8) and were stained with Coomassie brilliant blue R (Sigma Chemical Company, St. Louis, Missouri, U.S.A.). Alternatively, the resolved proteins were transferred to nitrocellulose (25) and probed with antibodies as described above. An anti-β-galactosidase mouse monoclonal antibody was purchased from Promega.
Affinity purification of rabbit antibodies with clone A. The λgt 11 clone A was plated at 2-5 x 104 phage in LB top agar on a 90- mm LB plate. Clone A plaques were lifted onto IPTG-treated nitrocellulose and processed as described above. The blots were incubated with the hyperimmune rabbit antiserum and after washes the bound antibodies were eluted from the antigen according to the procedure of Snyder, et al. (23). Sodium azide (0.01%) was added to the eluate to inhibit contamination.
Southern blots. Phage DNA was prepared according to a plate lysate procedure (19). Recombinant phage DNA was digested to completion with restriction endonucleases (EcoRI, Xhol; Promega) and electrophoresed in a 0.8% (w/v) agarose gel. The gel was stained with ethidium bromide and photographed. DNA was transferred to a PhotoGene nylon membrane (GIBCO BRL, Grand Island, New York, U.S.A.) by a standard capillary transfer technique (19) followed by UV-crosslinking (Stratalinker; Stratagene, La Jolla, California, U.S.A.) at 120,000 microjoules/cm2. Prehybridization of the membrane was performed essentially as described in Maniatis, et al. (19) for 4 hr at 42ºC, followed by overnight hybridization at 42ºC in the presence of 50 ng/ml denatured biotinylated probe per cm2 of membrane and 10% (w/v) dextran sulfate. Detection of the hybrids was performed with the PhotoGene nucleic acid detection system (GIBCO BRL) as described by Carlson, et al. (5) with a 5-7 min exposure to Kodak X-OMAT film. The blot was stripped in a solution of 95% formamide and 0.5% SDS at 65ºC for 1 hr.
Preparation of DNA probes. Plasmid DNA from pUC3, a pUC derivative constructed by Dr. T. P. Gillis, was prepared by standard procedures. This plasmid has at the EcoRI polylinker an ~2900-base pair (bp) DNA fragment subcloned from the M. leprae DNA of clone Y3178. Clone Y3178 DNA, prepared as described above, and the pUC3 DNA were digested with EcoRI. An ~3600-bp EcoRI-EcoRI fragment, containing the entire 65-kDa coding sequence and contiguous noncoding region of M. leprae DNA from clone Y3178, was isolated by agarose gel electrophoresis followed by electroelution. A second fragment, the ~2900bp EcoRI-EcoRI pUC3 DNA fragment corresponding to positions 53 to 2929 of clone Y3178 (20), containing the complete coding sequence of the 65-kDa gene, was similarly isolated. Both DNA fragments were labeled with biotin-ATP by nick-translation (BioNick; GIBCO BRL) and stored at -20ºC.
RESULTS
Screening of the λgt 11 M. leprae library and characterization of clone lysogen. Approximately 2.4 x 105 plaques from the λgt 1 1 library of M. leprae were screened for the expression of antigenic determinants with hyperimmune rabbit antiserum to M. leprae. Twenty-three reactive plaques were isolated by plugging with a sterile pasteur pipet. The reactive plaques were plaque purified (Fig. 1) and then immunoscreened by dot blot with monoclonal antibodies to some well-characterized antigens: IVD8, IIC8, IIH9, E42β, and Y1-2 to the 65-kDa protein, F47-9 to the 36-kDa protein, SA1D2D1 to the 28-kDa protein, L5 to the 18-kDa protein, ML06-A1 to the 12-kDa protein, and 46.7 to phenolic glycolipid (4,9, 10,). In all cases the clones selected with the rabbit antiserum were nonreactive with the murine monoclonal antibodies.
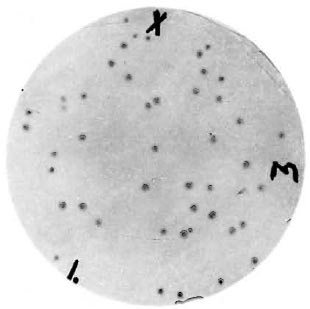
Fig. 1. Immunoblot of plaque purified clone A from the λgt11 M. leprae expression library. Plaque lifts were reacted with rabbit anti- M. leprae antiserum followed by an alkaline phosphatase-conjugated goat anti-rabbit antiserum. Markings were used to align the nitrocellulose filter with the plate.
E. coli lysogens that were constructed with recombinant bacteriophages were induced and the cell extracts examined by SDS-PAGE. Figure 2 shows a Coomassie blue stained SDS-polyacrylamide gel of the lysogens A, Y3180, Y3178, and Y3179. Lane A, the A lysogen, shows the presence of a high molecular weight protein of approximately 132-143-kDa. This suggests that the polypeptide specified by the recombinant clone A is a fusion protein with β-galactosidase. This protein is larger than the fusion protein of clone Y3180 (Fig. 2, lane 36) that expresses an epitope of the 36-kDa protein. The A fusion protein closely approximates the size of the fusion protein of clone Y3179 (Fig. 2, lane 18) that expresses the carboxy-terminal 111 amino acids of the 18-kDa protein (1). In order to determine if the high molecular weight protein was actually a fusion protein, a Western blot of clone A and clone Y3180 was probed with a monoclonal anti β-galactosidase antibody (data not shown). The demonstration of an immunorcactive protein of a high molecular weight confirmed that clone A encoded a fusion protein.

Fig 2. SDS-PAGE of lambda lysogens. Lysates of lambda lysogens (lane 65, clone Y3178; lane A, clone A; lane 36, clone Y3180; lane 18, clone Y3179) and nonlysogenized coli (lane, E. coli) were subjected to electrophoresis in a 10% polyacrylamide slab gel. The prestained standards (lane, STD) are: β-galactosidase, 116 kDa; phosphorylase B, 95.5 kDa; glutamate dehydrogenase, 55 kDa; ovalbumin, 43 kDa; lactate dehydrogenase, 36 kDa; carbonic anhydrase, 29 kDa; and lactoglobulin, 18.4 kDa (Diversified Biotech, Newton Centre, Massachusetts, U.S.A.).
Affinity-purified rabbit antibodies to the A fusion protein were prepared as described in Materials and Methods and used in a Western blot analysis against M. leprae cell extract to determine the size of the M. leprae protein represented by the epitope of the A fusion protein. Immunoblot analysis showed that the affinity-purified rabbit antibodies predominantly recognized a single polypeptide with an apparent molecular weight of 64 kDa. To confirm that this was not the well-characterized 65-kDa protein, the blot was repeated and half of the nitrocellulose was again probed with the affinity-purified antibodies (Fig. 3, blot A) and half of the strip was probed with the monoclonal antibody IVD8 to the 65-kDa protein (Fig. 3, blot IVD8). These immunoblots show that the M. leprae protein expressing the A epitope is smaller than the M. leprae 65-kDa protein. Additional demonstration that the immunologically reactive fusion protein from the A lysogen did not express an antigenic determinant of the 65-kDa protein was shown by Western blot analysis of the A lysogen with a battery of monoclonal antibodies to the 65-kDa protein. Five different monoclonal antibodies (IVD8, IIC8, E42β, Yl-2, and IIH9) reacted with the 65kDa protein from the Y3178 lysogen, but no reactivity was shown for the A fusion protein (Fig. 4). There was some crossreaction with E. coli proteins from both the test and control lysogens.
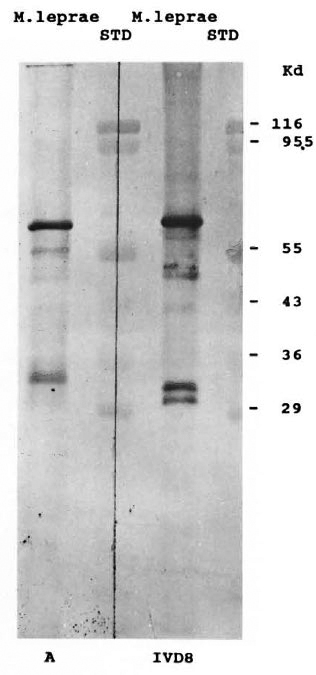
Fig. 3. Western blot with monospecific A antibodies. Aliquots of cell extracts of M. leprae and prestained molecular weight standards were subjected to electrophoresis as described in Fig. 2 and transferred to nitrocellulose paper. Blot A was incubated with monospecific hyperimmune rabbit scrum/affinity purified rabbit antibodies, washed and treated with-alkaline phosphatase-conjugated goat anti-rabbit IgG antiserum. Blot IVD8 was incubated with monoclonal antibody, IVD8, washed, and treated with an alkaline phosphatase-conjugated goat anti-mouse IgG antiserum. Standard lanes (STD) are as described in Fig. 2.
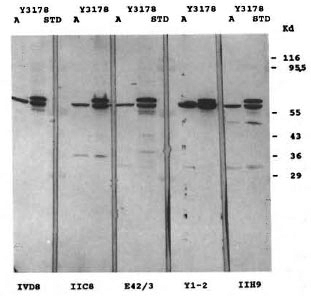
Fig. 4. Western blot with five different 65-kDa reactive monoclonal antibodies. Lysates of lambda lysogens (lanes A, clone A; lanes Y3178, clone Y3178) were subjected to electrophoresis as described in Fig. 2 and transferred to nitrocellulose paper. The paper was cut into strips and each strip incubated with a different monoclonal antibody, IVD8, IIC8, E42β, Yl2, and IIH9. The strips were washed, treated with an alkaline phosphatasc-conjugated goat anti-mouse IgG antiserum, and the color developed. Standard lanes (STD) are as described in Fig. 2.
Characterization of the M. leprae DNA in clone A. Clone A DNA was isolated from recombinant phage and cut with the restriction enzyme EcoRI in order to determine the size of the M. leprae DNA insert fragment. Besides the two lambda arms, two DNA pieces of approximately 3800 bp and 650 bp were present (Fig. 5A, lane 2) on a 0.8% agarose gel. The similarity in size of the large fragment of insert DNA to the EcoRI-EcoRI DNA fragment of the 65-kDa clone (Fig. 5A, lane 4) was cause for concern that clone A might contain the 65-kDa gene sequence. Partial EcoRI digests of clone A DNA demonstrate multiple bands of insert DNA, suggesting the presence of at least two internal EcoRI sites (data not shown). The largest DNA fragment after partial digestion was approximately 4450 bp; this reflects the total amount of M. leprae DNA in clone A.
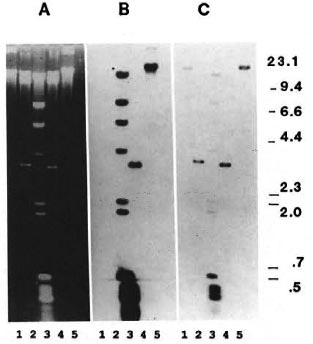
Fig. 5. Southern blot with two 65kDa specific probes: A = Ethidium-bromide-stained agarose gel; samples are: lane 1, clone A DNA-XhoI digest; lane 2, clone A DNA-EcoRI digest; lane 3, biotinylated XDNA-Hindlll fragments and biotinylated 0X174 DNA-Hinfl fragments (GI13CO BRL); lane 4, clone Y3178 DNA-EcoRI digest; and lane 5, clone Y3178 DNA-Xhol digest. B = DNA from Panel A was transferred to a nylon membrane and probed with nick translated 2900-bp Nrul-Nrul fragment from pUC3 containing the 65kDa coding sequences. C = Membrane in Panel B after stripping and reprobing with a nick-translated 3600-bp EcoRI fragment from clone Y3178.
EcoRI-digested phage DNA from both clone A and the recombinant 65-kDa clone (Y3178) were electrophoresed and transferred to a nylon membrane for Southern blotting with two different biotin-labeled probes encoding the 65-kDa protein. The first probe, 2900 bp, contained all of the DNA encoding the protein and 1100 bp of distal DNA. This probe hybridized to the 3600-bp DNA fragment from clone Y3178 as expected (Fig. 5B, lane 4), but did not hybridize to either the 3800-bp or 650-bp
DNA fragments from the recombinant clone A (Fig. 5B, lane 2). These fragments are visible in the ethidium-bromide-stained gel before transfer (Fig. 5A). Figure 5C shows the same blot after the membrane was stripped and reprobed with the second probe-the entire insert DNA from the recombinant clone Y3178. This probe contains an additional 700 bp of DNA from the noncoding regions of the 65-kDa clone; it hybridized to itself (Fig. 5C, lane 4) as expected and additionally hybridized to the larger DNA fragment from clone A (Fig. 5C, lane 2).
DISCUSSION
We have isolated clones from the M. leprae λgt 1 1 expression library of Young, et al. (33) with hyperimmune rabbit antiserum. These clones expressed protein products that were nonreactive with a panel of monoclonal antibodies. In this paper we have described clone A, that encodes a fusion protein of 132-143 kDa and expresses an antigenic determinant of a 64-kDa M. leprae protein. Western blot analyses with murine antibody IIH9, which maps to an epitope of the amino terminus of the 65-kDa protein, and monoclonal antibodies IIC8 and IVD8, which map to epitopes in the carboxy terminus of the 65-kDa protein (20), show the A fusion protein to be completely nonreactive. IVD8 recognizes the same M. leprae specific epitope as IIIE9 (4). Clone A is unique to those isolated thus far from the λgt 1 1 library in that it expresses an epitope of a 64-kDa protein and the DNA insert contains internal EcoRI sites.
The 64-kDa protein appears to be a major immunodominant antigen for rabbits immunized with M. leprae because additional clones that we have analyzed contain the same insert DNA. We suggest that the 64-kDa antigen of hi. leprae has not been described previously, and we plan to characterize our other reactive clones for additional antigenic determinants. Our preliminary studies have indicated that the A lysogen is reactive with some sera from leprosy patients when the A lysogen is used in an antibody-capture assay (unpublished data). Future studies to confirm this observation and the immunological role of the 64-kDa antigen in the immune response to M. leprae are planned.
Clone A DNA does share some homology with the 3600-bp DNA fragment of clone Y3178 but, interestingly enough, this is not due to homology with the repetitive sequence that has been identified in M. leprae (7,28). At least 27 copies of this repetitive sequence are present in the M. leprae genome and have been identified in the noncoding 3' region of the 65-kDa insert-DNA (bases 2127 to 2815), a region present in both of the probes used in our study. Perhaps the homology observed in our experiments is due to a different repetitive element in M. leprae DNA. Our smaller probe did not contain ~650 bp at the 3' end and also lacked the 5' sequences (bases 1 to 53) that are likely to contain the promoter region for the 65-kDa gene.
Based on the large size of the DNA fragment of clone A and the small size of the fusion protein, it is apparent that the majority of the insert DNA consists of M. leprae DNA not coding for the 64-kDa protein. The possibility remains that the noncoding region of the A insert DNA contains another open reading frame and another promoter region as found with a 3200-bp DNA fragment containing the gene for the 18-kDa protein (1). We are currently sequencing the insert DNA and will use computer programs to search for a repetitive region shared by the DNA fragments of clone Y3178 (20) and clone A.
Acknowledgments. We are grateful to Dr. Tom Gillis for discussions and sharing of materials critical for this investigation, to Dr. Ramachandra Navalkar for his generous gift of reagents for this study, and to Tonya Savage for manuscript preparation. This work was supported in part by NIH Grants S06GM08248 and 2G12RR03034 to SKD. Gregory T. George was an undergraduate student at Morehouse College, Atlanta, Georgia, U.S.A., during the course of this study.
REFERENCES
1. BOOTH, R. J., HARRIS, D. P., LOVE, J. M and WATSON, J. D. Antigenic proteins of Mycobacterium leprae: complete sequence of the gene for the 18-kDa protein. J. Immunol. 140(1988)597-601.
2. BRITTON, W. J., GARSIA, R. J., HELLQVIST, L., WATSON, J. D. and BASTEN, A. The characterization and immunoreactivity of a 70 K.D protein common to Mycobacterium leprae and Mycobacterium bovis (BCG). Lepr. Rev. 57 Suppl. 2 (1986)67-75.
3. BRITTON, W. J., HELLQVIST, L., GARSIA, R. J. and BASTEN, A. Dominant cell wall proteins of Mycobacterium leprae recognized by monoclonal antibodies. Clin. Exp. Immunol. 67(1987)31-42.
4. BUCHANAN, T. M., NOMAGUCHI, H., ANDERSON, D. C, YOUNG, R. A., GILLIS, T. P., BRITTON, W. J., IVANYI, J., KOLK, A. H. J., CLOSS, O., BLOOM, B. R. and MEHRA, V. Characterization of antibody-reactive epitopes on the 65-kilodalton protein of Alycobacterium leprae. Infect. Immun. 55(1987)1000-1003.
5. CARLSON, D. P., SUPERKO, C, MACKEY, J., GAS-KILL, M. E. and HANSEN, P. Chemiluminesccnt detection of nucleic acid hybridization. Focus 12(1990)9-12.
6. CHERAYIL, B. J. and YOUNG, R. A. A 28-kDa protein from Alycobacterium leprae is a target of the human antibody response in lepromatous leprosy. J. Immunol. 141(1988)4370-4375.
7. CLARK-CURTISS, J. E. and DOCHERTY, M. A. A species-specific repetitive sequence in Alycobacterium leprae DNA. J. Infect. Dis. 159(1989)7-15.
8. CLARK-CURTISS, J. E., JACOBS, W. R., DOCHERTY, M. A., RITCHIE, L. R. and CURTISS, R., III. Molecular analysis of DNA and construction of genomic libraries of Alycobacterium leprae. J. Bacteriology 161(1985)1093-1102.
9. ENGERS, H. D., ABE, M., BLOOM, B. R., MEHRA, V., BRITTON, W., BUCHANAN, T. M., KHANOLKAR, S. K., YOUNG, D. B., CLOSS, O., GILLIS, T., HARBOE, M., IVANYI, J., KOLK, A. H. J. and SHEPARD, C. C. Results of a World Health Organization-sponsored workshop on monoclonal antibodies to Mycobacterium leprae. (Letter) Infect. Immun. 48(1985)603-605.
10. ENGERS, H. D., HOUBA, V., BENNEDSEN, J., BUCHANAN, T. M., CHAPARAS, S. D., KADIVAL, G., CLOSS, O., DAVID, J. R., VAN EMBDEN, J. D. A., GODAL, T., MUSTAFA, S. A., IVANYI, J., YOUNG, D. B., KAUFMANN, S. H. E., KHOMENKO, A. G., KOLK, A. H. J., KUBIN, M., LOUIS, J. A., MINDEN, P., SHINNICK, T. M., TRNKA, L. and OUNG, R. A. Results of a World Health Organization-sponsored workshop to characterize antigens recognized by Atycobacterium-specific monoclonal antibodies. (Letter) Infect. Immun. 51(1986)718-720.
11. HARTSKEERL, R. A., VA N RENS, R. M., STABEL, L. F. E. M., DE WIT, M. Y. L. and KLATSER, P. R. Selection and characterization of recombinant clones that produce Alycobacterium leprae antigens recognized by antibodies in sera from household contacts of leprosy patients. Infect. Immun. 58(1990)2821-2827.
12. HUNTER, S. W., RIVOIRE, B., MEHRA, V., BLOOM, B. R. and BRENNAN, P. J. The major native proteins of the leprosy bacillus. J. Biol. Chem. 265(1990)14065-14068.
13. HUYNH, T. V., YOUNG, R. A. and DAVIS, R. W. Constructing and screening cDNA libraries in λgt10 and λgt 1 1. In: DNA Cloning: .1 Practical Approach,Vol. 1. Glover, D. M., ed. Oxford: IRL Press, 1985,pp. 49-78.
14. IBRAHIM, M. A., LAMB, F. I. and COLSTON, M. J. Analysis of variation in batches of armadillo-derived Mycobacterium leprae by immunoblotting.Int. J. Lepr. 58(1990)73-77.
15. JACOBS, W. R., DOCHERTY, M. A., CURTISS, R., III,and CLARK-CURTISS, J. E. Expression of Mycobacterium leprae genes from a Streptococcus mutans promoter in Escherichia coli K-12. Proc. Natl.Acad. Sci. U.S.A. 83(1986)1926-1930.
16. KHAN, M. B., DESIIPANDE, R. G., DAVIDSON, S. K.and NAVALKAR, R. G. Sero-immunoreactivity ofcloned protein antigens of Mycobacterium leprae.Int. J. Lepr. 60(1992)195-200.
17. LAAL, S., SHARMA, Y. D., PRASAD, H. K., MURTAZA,A., SINGH, S., TANGRI, S., MISRA, R. S. and NATII, I. Recombinant fusion protein identified by lepromatous sera mimics native Mycobacterium leprae in T-cell responses across the leprosy spectrum. Proc. Natl. Acad. Sci. U.S.A. 88(1991)1054-1058.
18. LAEMMLI, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(1970)680-685.
19. MANIATIS, T., FRITSCH, E. F. and SAMBROOK, J. Molecular Cloning, a Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press, 1982.
20. MEHRA, V., SWEETSER, D. and YOUNG, R. A. Efficient mapping of protein antigenic determinants. Proc. Natl. Acad. Sci. U.S.A. 83(1986)7013-7017.
21. SATHISH, M., ESSER, R. E., THOLE, J. E. R. and CLARK-CURTISS, J. E. Identification and characterization of antigenic determinants of Mycobacterium leprae that react with antibodies in sera of leprosy patients. Infect. Immun. 58(1990)1327-1336.
22. SHINNICK, T. M., VODKIN, M. H. and WILLIAMS, J. C. The Mvcobacterium tuberculosis 65-kilo-dalton antigen is a heat shock protein which cor-responds to common antigen and to the Esche-richia colt GroEL protein. Infect. Immun. 56(1988)446-451.
23. SNYDER, M., ELLEDGE, S., SWEETSER, D., YOUNG, R. A. and DAVIS, R. W. λgt 1 1: gene isolation with antibody probes and other applications. Meth. Enz.154(1987)107-128.
24. THOLE, J. E. R., STABEL, L. F. E. M., SUYKERBUYK, M. E. G., DE WIT, M. Y. L., KLATSER, P. R., KOLK,A. H. J. and HARTSKEERL, R. A. A major immunogenic 36,000-molecular-weight antigen from Mycobacterium leprae contains an im munoreaclive region of proline-rich repeats. Infect. Immun.58(1990)80-87.
25. TOWBIN, H., STAEHELIN, T. and GORDON, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure andsome applications. Proc. Natl. Acad. Sci. U.S.A.76(1979)4350-4354.
26. VADIEE, A. R., SHANNON, E. J., GILLIS, T. P. and HASTINGS, R. C. Partial characterization of antigens from M. leprae evoking IgG and 1gM antibodies in armadillos. Int. J. Lepr. 56(1988)274-282.
27. VEGA-LOPEZ, F., BROOKS, L. A., DOCKRELL, H. M.,DE SMET, K. A. L., THOMPSON, J. K., HUSSAIN, R. and STOKER, N. G. Sequence and immunological characterization of a serine-rich antigen from Mycobacterium leprae . Infect. Immun. 61(1993)2145-2153.
28. WOODS, S. A. and COLE, S. T. A family of dispersed repeats in Mycobacterium leprae . Mol. Microbiol. 4(1990)1745-1751.
29. YOUNG, D., LATHIGRA, R., HENDRIX, R., SWEETSER, D. and YOUNG, R. A. Stress proteins areimmune targets in leprosy and tuberculosis. Proc.Natl. Acad. Sci. U.S.A. 85(1988)4267-4270.
30. YOUNG, D. B., FOHN, M. J., KHANOLKAR, S. R. and BUCHANAN, T. M. Monoclonal antibodies toa 28,000 mol. wt protein antigen of Mycobacterium leprae . Clin. Exp. Immunol. 60(1985)546-552.
31. YOUNG, D. B., KENT, L. and YOUNG, R. A. Screening of a recombinant mycobacterial DNA library with polyclonal antiserum and molecular weight analysis of expressed antigens. Infect. Immun. 55(1987)1421-1425.
32. YOUNG, R. A. and DAVIS, R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science 222(1983)778-782.
33. YOUNG, R. A., MEHRA, V., SWEETSER, D.,BUCHANAN, T., CLARK-CURTISS, J., DAVIS, R. W. and BLooM, B. R. Genes for the major proteinantigens of the leprosy parasite Mycobacterium leprae . Nature 316(1985)450-452.
1. Ph.D.; Department of Microbiology and Immunology, Morehouse School of Medicine, 720 Westview Drive SW, Atlanta, Georgia 30310, U.S.A.
2. Department of Microbiology and Immunology, Morehouse School of Medicine, 720 Westview Drive SW, Atlanta, Georgia 30310, U.S.A.
Received for publication on 9 August 1993.
Accepted for publication in revised form on 4 January 1994.