- Volume 62 , Number 2
- Page: 245–55
Antibody response to recombinant 65-kDa, 70-kDa and 18-kDa mycobacterial antigens in leprosy patients and healthy contacts in a leprosy-endemic population
ABSTRACT
Antibody responses to recombinant Mycobacterium leprae 65-kDa (rML65) and 18kDa (rMLl8), M. bovis BCG 65-kDa (rMB65) and M. tuberculosis 70-kDa (rMT70) antigens were measured by indirect ELISA in sera f rom leprosy patients and healthy contacts in a leprosy-endemic area in southern India. Antibody responses to M. leprae -specific epitopes on phenolic glycolipid-I (PGL-I) and a 35-kDa protein antigens also were measured simultaneously by PGL-I ELISA and the serum antibody competition test (SACT), respectively. Significantly higher levels of antibodies of the IgG isotype to rML65 and rMB65 were observed in bacterial index (Bl)-positive, lepromatous (LBI+) patients but not in other groups of leprosy patients and endemic controls [healthy family contacts (HFC), healthy hospital contacts (HHC), and healthy noncontacts (HNC)]. LBI+ patients could be distinguished f rom LBI- patients on the basis of their higher levels of IgG antibodies to rML 65, rMB65 and rMT70; lower levels of IgM antibodies to these antigens and higher levels of anti-PGL-I IgM levels. In the former group, 84% were SACT positive in contrast to 39% in the latter groups. Among lepromatous patients good positive correlations were observed between IgG antibody responses to rML65 and rMB65 and anti-PGL-I IgM levels, SACT ID50 titers as well as Bis. Among healthy controls, HFC had higher levels of IgG antibodies to rML65, but lower levels to rMB65 than did HNC. Thirty-nine percent of the HFC were seropositive to anti-PGL-I IgM antibodies in contrast to 4% in the HNC. On the basis of these criteria, the immune profile of the HFC appears to be distinctly different f rom that of the HNC, even though both groups are f rom the same endemic area. It is therefore possible that antibody response to defined protein antigens of mycobacteria is influenced by the lesional bacterial load in leprosy patients and by exposure to homologous proteins of M. leprae and/or related environmental mycobacteria in the case of healthy contacts and noncontacts. The above results are discussed in relation to T- and B-cell activity toward M. leprae antigens and the immunoregulatory mechanisms of antibody production in leprosy.RÉSUMÉ
Les réponses en anticorps vis-à-vis des gènes recombinants de 65 kDa (rML65) et 18 kDa (rML18) de Mycobacterium leprae, de 65 kDa du BCG de M. bovis (rMB65), et de 70 kDa de M. tuberculosis (rMT70) ont été mesurées par un ELISA indirect sur des sérums de malades de la lèpre et de contacts en bonne santé dans une région où la lèpre est endémique dans le Sud de l'Inde. Les réponses en anticorps au glycolipide phénolique I (PGL-I) spécifique à M. leprae et à des antigènes d'une protéine de 65kDa ont été également mesurées simultanément respectivement par ELISA et le test de compétition des anticorps sériques (SACT). Des taux significativement plus élevés d'anticorps de l'isotype IgG vis-à-vis du rML65 et du rMB65 ont été observés chez des patients lépromateux avec un indice bactérien positif (LBI + ), mais pas dans d'autres groupes de patients lépreux et de témoins de zones endémiques [contacts familiaux en bonne santé (CFS), des contacts d'hôpital en bonne santé (CHS) et des non-contacts en bonne santé (NCS)]. Les patients LBI+ pouvaient être distingués des patients LB- sur base de leurs taux plus élevés d'anticorps IgG vis-à-vis du rML65, rMB65 et rMT70, des taux plus faibles d'anticorps IgM vis-à-vis de ces antigènes, et des taux plus élevés d'IgM anti-PGL-I. Dans le premier groupe, 84% étaient positifs au SACT, par contraste avec 39% dans les autres groupes. Parmi les patients lépromateux, on a observé de bonnes correlations positives entre les réponses d'anticorps IgG vis-à-vis du rML65 et rMB65 et les taux d'IgM anti-PGL-I, les titres ID50 du SACT ainsi que l'indice bactérien. Parmi les témoins en bonne santé, les CFS avaient des taux d'anticorps IgG plus élevés vis-à-vis du rML65, mais plus bas vis-à-vis du rMB65 que n'avaient les NCS. Trente-neuf pourcents des CFS étaient séropositifs pour les anticorps anti-PGL-I, par contraste à 4% chez les NCS. Sur base de ces critères, le profil immunitaire des CFS apparaît distinct de celui des NCS, même si les deux groupes proviennent de la même zone endémique. Il est done possible que la réponse en anticorps à des antigènes protéiques bien définis de mycobactéries soit influencée par la charge bactérienne des lésions ches les malades de la lèpre et par l'exposition à des protéines homologues de M. leprae et/ou de mycobactéries apparentées de l'environnement dans le cas de contacts et non-contacts en bonne santé. Les résultats ci-dessus sont discutes en relation avec l'activité des cellules T et B vis-à-vis des antigènes de M. leprae et des mécanismes de régulation immunitaire de la production d'anticorps dans la lèpre.RESUMEN
Ultilizando un ELISA indirecto se midió la reactividad de los sueros de pacientes con lepra y de un grupo de controles sanos de un área endémica de lepra, al sur de la India. Como antígenos se usaron las proteínas recombinantes de 65 kDa de Mycobacterium leprae (rML65), de 65 kDa de M. bovis, BCG (rMB65), de 70 kDa de M. tuberculosis (rMT70). También se midieron las respuestas en anticuerpos contra el glicolípido fenólico-I (PGL-I) y contra una proteína de 35 kDa de M. leprae. Para ésto, se utilizaron un ELISA-PGL-I y una prueba de competencia de anticuerpo (SACT), respectivamente. En los pacientes lepromatosos con índices bacteriológicos positivos (LBI + ), se encontraron niveles muy elevados de anticuerpos IgG contra rML65 y contra rMB65. Esto no se observó en otros grupos de pacientes con lepra ni en los controles de la zona endémica: contactos familiares sanos (HFC), contactos sanos de hospital (HHC), y controles sanos no contactos (HNC). Los pacientes LBI+ pudieron distinguirse de los pacientes LBI- en base a sus niveles más altos de anticuerpos IgG contra rML65, rMB65, y rMT70, a sus mas bajos niveles de anticuerpos IgM contra estos antígenos, y a sus mayores niveles de anticuerpos IgM contra el PGL-I. El ochenta y cuatro porciento de los pacientes LBI+ fueron SACT positivos en contraste con el 39% de los pacientes LBI-. En los pacientes lepromatosos se observaron buenas correlaciones positivas en cuanto a sus respuestas en anticuerpos IgG contra rML65 y rMB65, en sus niveles de anticuerpos IgM contra el PGL-I, en sus títulos SACT ID50 y en sus Bis. Entre los controles sanos, los HFC tuvieron niveles más altos de anticuerpos IgG contra rML65 y niveles más bajos contra rMB65, que los HNC. Treinta y nueve porciento de los HTC, contra el 4% de los HNC, tuvieron anticuerpos IgM anti-PGL-I. Con base en estos hallazgos, el perfil inmune de los HFC resulta claramente diferente del perfil de los HNC, aún cuando ambos grupos sean de la misma zona endémica. Por lo tanto, es posible que la respuesta en anticuerpos contra antigenos proteicos definidos de las micobacterias esté inlluencíada por la carga bacteriana lesional de los pacientes con lepra y por la exposición a proteínas homologas de M. leprae y/o de otras micobacterias ambientales en el caso de los contactos y los no contactos sanos. Los resultados anteriores se discuten en relación a la actividad las células T y las células B contra los antígenos de M. leprae. y de los mecanismos de regulación de la producción de anticuerpos en la lepra.The availability of monoclonal antibodies to mycobacterial antigens has greatly helped in the characterization of the immunogenic components of Mycobacterium leprae (4, 7, 11,). Identification of species-specific epitopes on certain M. leprae antigens, such as phenolic glycolipid-I (PGL-I) and 35-kDa and 36-kDa protein antigens, have led to the development of specific serological tests for leprosy (14,25,32). Even though these tests have failed to achieve the goal of early detection of leprosy because of their limited specificity and/or sensitivity (21,26), their contribution toward our understanding of the immune response to M. leprae is significant.
Advances in molecular biology and the cloning of M. leprae and other mycobacterial genes have resulted in the availability of recombinant antigens in pure form to carry out detailed immunological investigations (32 ,33 ). A few of these antigens, such as 65-kDa, 70-kDa, 30/31 -kDa and 18-kDa proteins, have been studied in considerable detail (29). However, only a limited number of studies have been reported on antibody responses to 65-kDa (13, 16, 18), 70-kDa (3), 18-kDa (13,20) and 30/31-kDa (5) antigens in leprosy patients and healthy contacts in leprosy-endemic populations. Such population studies involving the defined antigenic constituents of M. leprae would help the efforts to develop specific sérodiagnostic tests for leprosy. Further, these studies will be useful in understanding the immunoregulatory mechanisms of antibody production in leprosy.
In this study we have measured antibodies of IgG and IgM isotypes to certain recombinant antigens, i.e., M. leprae 65-kDa and 18-kDa antigens, M. bovis BCG 65-kDa antigen and M. tuberculosis 70-kDa antigen, in sera from leprosy patients across the clinical spectrum and contacts of leprosy patients from a leprosy-endemic area in southern India. A PGL-I ELISA and the serum antibody competition test (SACT) that measures antibody response to M. leprae specific epitopes on PGL-I and a 35-kDa protein antigens were also carried out simultaneously.
MATERIALS AND METHODS
Antigens and monoclonal antibodies (MAbs). Recombinant M. leprae 65-kDa (rML65), M. bovis BCG 65-kDa (rMB65), M. tuberculosis 70-kDa (rMT70) and M. leprae 18-kDa (rML18) antigens were generously supplied by Dr. J. D. A. van Embden, Bilthoven, The Netherlands. MAbs Cl.l and IIC.8 (recognize crossreactive epitopes on mycobacterial 65-kDa antigen), HIE.9 (recognizes M. leprae -specific epitope on 65-kDa antigen) and L5 (against the M. leprae -specific epitope on 18-kDa antigen) were obtained from Dr. T. Shinnick, Centers for Disease Control, Atlanta, Georgia, U.S.A. Both recombinant antigens and MAbs were supplied through the UNDP/ World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR). Soluble M. leprae antigen (Leprosin A, batch CD 141), natural disaccharide of PGL-I conjugated to BSA (ND-O-BSA) and unconjugated BSA of the same lot were obtained from Dr. R. J. W. Rees (WHO/ IMMLEP/M. leprae Bank). MAb ML04 conjugated to horseradish peroxidase (ML04-HRP) was a kind gift from Dr. J. Ivanyi, Hammersmith Hospital, London, U.K.
Serum samples. Serum samples from leprosy patients, healthy family contacts (HFC), and healthy hospital contacts (HHC) were collected from a leprosy hospital (Voluntary Health Services, Leprosy Project) located at Sakthinagar in Periyar District, Tamil Nadu, India. The leprosy patients were classified clinically and bactcriologically (19) into polar lepromatous (LL), borderline lepromatous (BL), midborderline (BB), borderline tuberculoid (BT), and polar tuberculoid (TT) patients. HFC were healthy individuals living in the household of leprosy patients. Healthy hospital staff exposed to leprosy patients for 1 to 10 years' duration were classified as HHC. A total of 235 samples, collected from 151 leprosy patients (56 LL, 19 BL, 18 BB, 36 BT and 22 TT), 67 HFC, and 17 HHC were studied. LL and BL patients were grouped together and segregated into bacterial index (BI) positive (LBI + ) and BI negative (LBI-) lepromatous patients. Forty-seven healthy noncontact (HNC) samples were derived from students of the School of Biological Sciences, Madurai Kamaraj University, who did not have any habitual contact with leprosy patients. Serum separated from 4 ml of venous blood, collected in a siliconized vacuette (Vacuette; Griener, Germany) was decomplemented and frozen at - 70ºC.
Indirect ELISA for recombinant antigens. The antibody response to recombinant mycobacterial antigens was measured by an indirect ELISA protocol adopted from Meeker, et al. (18). ELISA plates (Corning Glassworks, Corning, New York, U.S.A.) were coated in duplicate with recombinant antigens diluted in 50 raM Tris buffer pH 8.0 (50 µl/well). Optimal coating concentrations for 65-kDa antigens (4 µg/ml) and rML 18 (2 µg/rnl) were determined using appropriate MAbs. rMT70 was used at a 4 /ug/ ml concentration. The plates were incubated at 37ºC for 1 hr and then at 4ºC overnight in a humidified chamber. The plates were washed twice with phosphate-buffered saline (PBS) containing 0.05% Tween-20 (PBST) and the wells were blocked with 1% gelatin (Sigma) in PBS (200Ml/well) for 1 hr at 37ºC. After washing, appropriate dilutions of serum samples (1:100 for IgG response; 1:20 for IgM response) in PBST containing 1% gelatin were added to the wells (50 µl/well). The optimal serum dilutions were determined using pooled sera from 50 normal subjects (NSP) or 50 lepromatous patients (LSP). After a 1-hr incubation at 37ºC, the plates were washed three times with PBST and appropriate dilutions of horseradish peroxidase (HRP) conjugated anti-human IgG or anti-human IgM (Cappel, Belgium) in PBST containing 1 % gelatin were added (50 µl/well) . The plates were incubated at 37ºC for 45 min, washed thoroughly, and freshly prepared OPD substrate solution was added (50 µl/well). After incubation at room temperature for 10 min in the dark, the enzyme reaction was stopped by the addition of 2 N H2S04 (50 µl/well). Absorbance was measured at 490 nm using a Dynatech Minireader II (Dynatech Laboratories Inc., Alexandria, Virginia, U.S.A.). NSP and LSP were included every time the ELISA was performed, and the coefficient of variation for eight separate assays was less than 10%.
Inhibition of MAb binding by human sera. Antibodies in leprosy patients' sera against certain MAb-defined epitopes on the recombinant antigens were measured by an inhibition ELISA technique. The antigencoated plates were first incubated with 1:20 dilution of the test serum for 1 hr at 37ºC. After washing, the plates were incubated with the appropriate MAb for 1 hr at 37ºC followed by anti-mouse IgG-HRP (Cappel, Belgium). Subsequent steps were the same as described above.
PGL-I ELISA. The method described by Sinha, et al. (24) was followed with minor modifications. Duplicate ELISA plates were coated with ND-O-BSA or the corresponding batch of BSA at a concentration of 0.1 µg/ml in 0.05 M carbonate buffer (pH 9.6) for 1 hr at 37ºC and then overnight at 4ºC (50 µl/well). The plates were washed with PBST and blocked with 1% BSA in PBST (BSA-PBST, 100 µl/well) for 1 hr at 37ºC. After washing a 1:300 dilution of the serum samples in BSA-PBST were incubated in duplicate wells for 1 hr at 37ºC (50 µl/well). After washing three times with PBST, the plates were incubated for 45 min at 37ºC with HRP-labeled anti-human IgM diluted in BSA-PBST (50µl/well). Subsequent steps were similar to those described for recombinant antigens. For each serum sample, the mean OD of BSA-coated wells was subtracted from the mean OD of ND-O-BSAcoated wells. Antigen-coated wells without test serum served as blanks. Samples with OD values above the mean + 2 S.D. value of healthy noncontacts (> 0.20) were considered positive.
Serum antibody competition test (SACT). Antibody response to a species-specific determinant (My2) on the 35-kDa M. leprae protein was determined by inhibition ELISA essentially as described by Sinha, et al. (24). Inhibition of the specific MAb (ML04-HRP) binding by serum antibodies was measured. Each serum sample was tested over serial fivefold dilutions from 1:5 to 1:625. The binding of ML04-HRP to antigen (Leprosin A, 50 µg/ml)-coated wells in the absence of serum gave the 100% binding value. Relative binding of ML04-HRP in wells incubated with different serum dilutions were calculated using the 100% binding value for each plate. Percent inhibition values were derived from these values, and the highest dilution of the test serum which showed > 50% inhibition of the 100% binding value (ID50 titer) was determined. A serum sample was considered SACT positive when the 5 0 titer was more than 1:5.
Statistical analysis. Student's / test and regression analysis were performed using the EPISTAT statistical package.
RESULTS
IgG responses to recombinant antigens. LBI+ patients showed significantly high levels of IgG antibodies to rML65 and rMB65 compared to other groups of leprosy patients, HFC, HHC and HNC (Fig. 1). Interestingly, LBI- patients had significantly lower levels of serum IgG to rML65, rMB65 and rMT70 than LBI+ cases. BB, BT and TT patients were comparable to HNC in their IgG responses to all the four antigens. There were wide individual variations in serum IgG levels to rML65, rMB65 and rMT70 in all groups of leprosy patients. Analysis of pooled data from LBI+ and LBI- patients showed a significant positive correlation between the IgG antibody levels to rML65 and rMB65 (r = 0.797, p < 10-6).
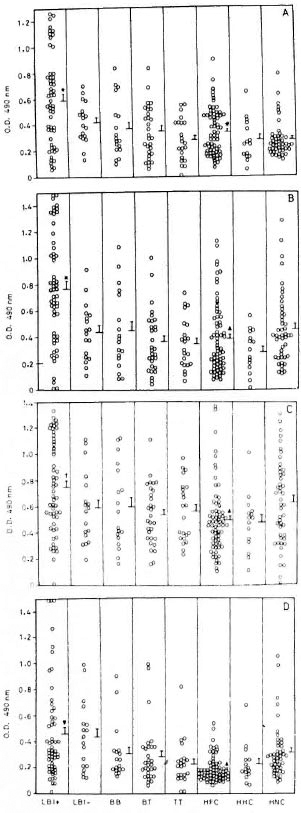
Fig 1. IgG response to recombinant (A) M. leprae 65-kDa (rML65), (B) M. bovis BCG 65-kDa (rMB65), (C) M. tuberculosis 70-kDa (rMT70) and (D) M. leprae 18-kDa (rMLl 8) antigens in different groups of leprosy patients, healthy contacts, and healthy noncontacts. Serum samples from 56 BI positive lepromatous (LB1 + ), 18 BI-negative lepromatous (LBI-), 18 midborderline (BB), 30 borderline tuberculoid (BT), and 22 polar tuberculoid (TT) leprosy patients, 67 healthyfamily contacts (HFC), 15 healthy hospital contacts(HHC), and 47 healthy noncontacts (HNC) were tested.
ELISA plates were coated with 4 pg/ml of rML65,rMB65 or rMT70, or 2 pg/ml of rML18, and incubatedwith 1:100 dilution of serum samples followed by anti-human IgG-HRP at 1:2000 dilution. Mean + S.E. ofthe response for each study group is marked. * = Meanvalue significantly higher than all other study groups(p values varied from 0.05 to 10-6): t = Mean valuesignificantly higher than all groups except HNC (p <0.05 to 10-4); ▼ = mean value significantly higher thanall the groups except LBI- (p < 0.05 to 10-6); # =mean value significantly higher than HNC (p < 0.05); ▲= mean value significantly low compared to HNC(p < 0.05).
HNC showed low levels of anti-rML65 IgG antibodies. However, their seroreactivity toward rMB65 and rMT70 were relatively high and widely scattered (Fig. 1). Interestingly, HFC showed significantly higher levels of anti-rML65 IgG antibodies than HNC. In contrast, their IgG responses to rMB65, rMT70 and rML18 were lower than that of HNC. On the other hand, HHC did not differ from HNC in their IgG responses to all the four antigens. These results indicate that HFC are distinct from HNC and HHC in terms of their anti-rML65 IgG response.
IgM response to recombinant antigens. In contrast to their IgG responses, the IgM levels of LBI+ patients to rML65, rMB65 and rMT70 were comparable to those of other study groups and, in fact, were significantly lower than those of LBI - patients (Fig. 2). IgM responses of HNC to rMB65 and rMT70 were not as widely scattered as their IgG responses to these antigens. HFC and tuberculoid patients showed wide variations in their IgM responses to rML18 compared to their IgG levels to the same antigen.
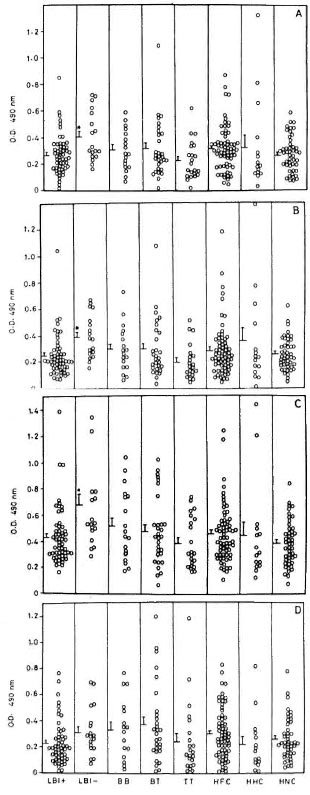
Fig. 2. IgM responses to (A) rML65, (B) rMB65,(C) rMT70 and (D) rML18 in different groups of leprosy patients, healthy contacts and healthy noncontacts. Serum samples were used at 1:20 dilution followed by anti-human IgM-HRP at 1:1000 dilution. Other details are the same as in Fig. 1. * = Meanresponse significantly higher than LBI+, TT and HNC(p < 0.05).
Anti-PGL-I IgM response. LBI+ patients showed markedly high levels of anti-PGL-I IgM antibodies compared to all other study groups including LBI- patients (Fig. 3). The proportion of positive cases declined gradually across the leprosy spectrum (LBI+ 82%, LBI- 56%, BB 50%, BT 53%, TT 36%). A considerable proportion of the contacts also showed positive anti-PGL-I IgM responses (HFC 39%, HHC 33%) even though their mean responses were not significantly different from those of the HNC who showed only 4% seropositivity. However, except for the LBI+ patients, the positive responders of all other patient groups and contacts were only marginally above the cut-off value. Analysis of the pooled data from lepromatous patients revealed that their anti-PGL-I IgM responses showed a strong positive correlation to the BI (r = 0.726, p < 10-6) and a negative correlation to the duration of multidrug chemotherapy (r = 0.411, p < 0.001).
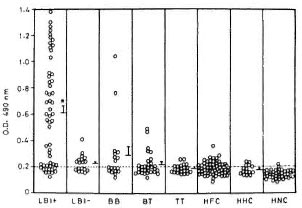
Fig. 3. Anti-PGL-I IgM response to PGL-I in various groups of leprosy patients, healthy contacts and healthy noncontacts. ELISA plates were coated with 0.1 µ of ND-O-BSA or BSA alone. Plates wereincubated with 1:300 dilution of each test serum fol-lowed by anti-human IgM-HRP at 1:1000 dilution. The mean absorbance value for BSA coated wells was subtracted from that of ND-O-BSA-coated wells foreach serum to obtain the value for antibodies reacting to M. lerprae-specific epitopes of PGL-I. Other detailsare the same as in Fig. 1. Dashed line represents cut-off limit for positive response, which is the mean + 2S.D. of HNC. * = Mean value significantly higher thanall other study groups (p < 0.05).
SACT profile. Antibody response to a species-specific epitope on the 35-kDa protein of M. leprae was measured by SACT. The highest proportion of SACT-positive cases was observed among the lepromatous patients, declining gradually toward the tuberculoid pole of the spectrum (Table 1). Further, 84% of the LBI+ patients were SACT positive in contrast to 39% of the LBI- patients. Moreover, the SACT ID50 titer was high among LBI+ patients, with more than 50% of the SACT-positive cases showing titers above 1:125. None of the SACT positive cases among the other patient groups showed such high ID50 titers. There was a strong positive correlation between the BI and the SACT ID50 titers among lepromatous patients (r = 0.637, p < 10-6 ). Even though their SACT ID50 titers showed a tendency to decline with the increase in the duration of chemotherapy, the correlation was not statistically significant (r = 0.220, p = 0.06).
Comparison of the lepromatous patients' IgG response to recombinant antigens with clinical and other serological parameters. Analysis of the pooled data from the LBI + and LBI- patients revealed that IgG antibody levels to rML65 and rMB65, but not to rMLl8, showed significant positive correlation to the Bis of the patients (Fig. 4). The correlation was only marginal between anti-rMT70 IgG response and the BI. However, when compared to the duration of multidrug chemotherapy, only the antirML65 IgG response showed a marginally significant negative correlation (Table 2).
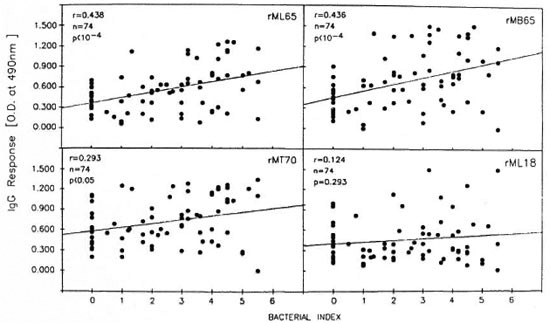
Fig. 4. Correlation between the BI and IgG responses to rML65, rMB65, rMT70 or rML18 in lepromatous patients. Data from LBI+ and LBI– patients were pooled for analysis (N = 74).
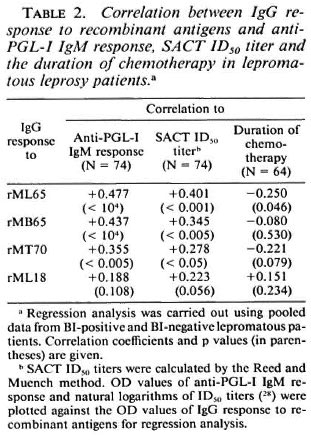
IgG antibody responses to rML65, rMB65 and rMT70 showed significant positive correlation to anti-PGL-I IgM responses and SACT ID50 titers (Table 2). However, antirML18 IgG responses did not show any correlation with anti-PGL-I IgM levels or the SACT ID50 titers.
Failure of inhibition of the MAb binding to rML65 and rML18 by human serum antibodies. The binding of MAbs C1.1, IIC.8 and IIIE.9 to rML65 and L5 to rMLl8 were measured following prior incubation with sera containing high levels of IgG antibodies to these antigens. None of the test sera from leprosy patients, HFC, HHC, and HNC inhibited the MAb binding (data not shown).
DISCUSSION
We have demonstrated earlier the presence of high levels of antimycobacterial antibodies of the IgG isotype in untreated LBI+ patients (17). Following effective chemotherapy such antibodies were significantly reduced in LBI- patients. The above study was carried out using the crude preparation of M. leprae sonicate supernatant. However, it is considered more appropriate to make use of the purified recombinant protein antigens of mycobacteria for a better understanding of the immunologically relevant antigens on the one hand and the immune profile of leprosy patients and healthy endemic controls on the other. Moreover, such a study would contribute toward the elucidation of the immunoregulatory mechanisms of the humoral immune response in leprosy.
The present study identifies several differences between BI+ and BI- lepromatous patients in their immune profile: a) IgG antibodies to 65-kDa mycobacterial proteins were significantly elevated in LBI + patients; however, antibodies of the IgM isotype to these antigens were at lower levels in BI+ than in BI- lepromatous patients; b) the anti-PGL-I IgM response and SACT 5 0 titer were much higher in LBI+ than in LBI- patients; c) there was a strong positive correlation between IgG antibody levels to certain recombinant mycobacterial antigens and the BI status among lepromatous patients and d) positive correlation was also observed between the IgG antibody response to these protein antigens and M. leprae-specihe anti-PGL-I IgM or SACT 5 0 titers. These results confirm earlier reports on the high levels of anti-65-kDa IgG (18) and anti-PGL-I IgM (24) antibodies in BI+ lepromatous patients, and demonstrate that, after effective chemotherapy when the bacterial load was reduced, con-comitant reduction in antibody response to mycobacterial antigens occurred.
Our data also suggest that the bacterial load in the lesions influence the IgG antibody response to M. leprae proteins. However, the underlying mechanism for the enhanced antibody production is not understood. It is interesting that such an enhancement occurs in lepromatous patients wherein the T-lymphocyte function which is essential for immunoglobulin class switch (15) has been shown to be poor (8-10). In this context our recent findings (manuscript submitted) on the inverse relationship between T-cell and B-cell responses to rML65 in individual leprosy patients and the control subjects would substantiate the role of lymphokines in immunoregulation (2, 23, 30) w hich might be responsible for enhanced antibody production in LBI+ patients to mycobacterial antigens and its concomitant reduction following effective chemotherapy.
Wide individual variations were observed in the IgG levels of each study group to rML65, rMB65 and rMT70. This is in sharp contrast to the anti-PGL-I IgM response and SACT ID50 titer wherein BI + lepromatous patients alone showed marked variation. It should be noted that while the PGL-I ELISA and SACT measured antibody responses to M. /eprae-specific epitopes, IgG responses measured against the recombinant antigens were polyspecific. The most plausible explanation for the observed scatter in IgG response to the recombinant proteins could be that these antigens belong to the family of heat-shock proteins which are highly conserved in several bacterial species (27). Moreover, these antigens have several epitopes crossreactive to other mycobacteria (1). Therefore, it is likely that most of the antibodies might be directed against the shared epitopes, thereby contributing significantly to the observed pattern of response.
HFC are distinct from HNC in their IgG responses to rML65, which could be attributed to the extensive exposure of the former to M. leprae. This is further substantiated by a significant proportion among them showing positive anti-PGL-I IgM levels. On the other hand, IgG responses to rMB6 5 and rMT70 were significantly higher in HNC than in HFC. Further, HNC showed higher responses to rMB65 than to rML65 (Fig. 1, p < 10-4), with wide individual variation. These subjects were derived from the student community in the university who did not have habitual contact with leprosy patients. Therefore, they are unlikely to have had extensive exposure to M. leprae, which would explain their relatively low levels of IgG responses to rML65 compared to rMB65 and their negligible anti-PGL-I IgM levels and SACT positivity. It is also possible that, even though ML65 and MB65 share extensive sequence homology (22), certain antibodies in HFC are directed against M. leprae -specific epitopes in rML65.
In addition to HFC, a considerable proportion of HHC also showed positive anti-PGL-I IgM levels. The fact that none among HFC or HHC was SACT positive suggests that antibody responses to surface glycolipid antigens and crossreactive epitopes on protein antigens appear to be elicited by the quantum of exposure to M. leprae, while antibody responses to M.leprae -specific epitopes on protein antigens appear to occur only after the establishment of active infection.
In order to investigate whether there is any qualitative difference between the patient groups, healthy contacts and HNC in the antibody responses to defined epitopes on rML65 and rML18, the binding of certain MAbs was measured following preincubation with sera showing high IgG response to these antigens. The binding of CI. 1 and IIC.8 to crossreactive epitopes on the 65-kDa antigen, and IIIE.9 and L.5 to M. leprae -specific epitopes on the 65-kDa and 18-kDa antigens, respectively, was not inhibited by any of the scrum samples tested. Similar findings also have been reported by others (11,28) who have used murine monoclonal antibodies to the 65-kDa antigen. It is, therefore, possible that the epitopes on the mycobacterial 65-kDa antigen recognized by human sera might be different from the ones defined by murine MAbs as suggested by Meeker, et al. (18). It should be noted that the 65-kDa and 70-kDa mycobacterial antigens, despite a high degree of conservation with the heat-shock proteins including their mammalian counterparts (6, 12, 31), are dominant B-cell immunogens in humans, as demonstrated by this study and other earlier reports (3, 13, 16, 18). Therefore, it may be useful to characterize the human B-cell epitopes on M. leprae protein antigens.
The present study on the profile of antibody responses to recombinant mycobacterial antigens demonstrates certain interesting features. Clearly, in lepromatous patients, their IgG antibody level to rML65 is reflective of the bacterial load in their lesions and thus can be used to monitor the effectiveness of chemotherapy. HFC certainly constitute a distinct group in terms of anti-rML65 IgG response. Detailed investigations into the antibody response, for example the IgG subclass levels to rML65, might throw light on the nature of T-cell help and the regulation of antibody production in leprosy patients.
Acknowledgment. This work was supported by grants from the Department of Biotechnology, Government of India, and Indo-Swiss Cooperations in Biotechnology. SI thanks the CSIR, Government of India, for the financial support in the form of a senior research fellowship.
REFERENCES
1. BUCHANAN, T. M., NOMAGUCHI, H., ANDERSON, D. C, YOUNG, R. A., GILLIS, T. P., BRITTON, W. J., IVANYI, J., K.OLK, A. H. J., CLOSS, O., BLOOM, B. R. and MEHRA, V. Characterization of antibody reactive epitopes on the 65-kilodalton protein of Mycobacterium leprae. Infect. Immun. 55(1987)1000-1003.
2. CHUJOR, C. S. N., RUHN, B., SCHWERER, B., BERNHEIMER, H., LEVIS, W. R. and BEVEC, D. Specific inhibition of mRNA accumulation for lymphokines in human T cell line Jurkat by mycobacterial lipoarabinomannan antigen. Clin. Exp. Immunol. 87 (1992)398-403.
3. DAVENPORT, MCKENZIE, K. R., BASTEN, A. and BRITTON, W. J. The variable C-terminal region of the Mycobacterium leprae 70-kilodalton heat shock protein is the target for humoral immune responses. Infect. Immun. 60(1992)1170-1177.
4. ENGERS, H. D., ABE, M. , BLOOM, B. R., MEHRA, V. , BRITTON, W., BUCHANAN, T. M. , KHANOLKAR, S. K., YOUNG, D. B., CLOSS, O., GILLIS, T., HARBOE, M. , IVANYI, J., KOLK, A. H. J. and SHEPARD, C. C. Results of a World Health Organization-sponsored workshop on monoclonal antibodies to M. leprae. (Utter) Infect. Immun. 48(1985)603-605.
5. ESPITIA, C, SCIUTTO, E., BOTTASSO, O., GONZALEZ AMARO , R., HERNANDEZ PANDO, R. and MANCILLA, R. High antibody levels to the mycobacterial fibronectin-binding antigen of 30-31 kD in tuberculosis and lepromatous leprosy. Clin. Exp. Immunol. 87(1992)362-367.
6. GARSIA, R. J., HELLQVIST, L., BOOTH, R. J., RAD FORD, A. J., BRITTON, W. J., ASTBURY, L., TRENT, R. J. and BASTEN, A. Homology of the 70-kilodalton antigens from Mycobacterium leprae and Mycobactrium bovis with the Mycobaclrium tuberculosis 71 -kilodalton antigen and with the conserved heat shock protein 70 of eucaryotes. Infect. Immun. 57(1989)204-212.
7. GAYLORD, H. and BRENNAN, P. J. Leprosy and the leprosy bacillus: recent advancements in characterization of antigens and immunology of the disease. Ann. Rev. Microbiol. 41(1987)645-675.
8. GODAL, T. Immunological spects of leprosypresent status. Prog. Allergy 25(1978)211-242.
9. GULLE, H., SCHOEL, B., CHIPLUNKAR, S., GANGAL, S., DEO, M. G. and KAUFMANN, S. H. E. T-cell responses of leprosy patients and healthy contacts toward separated protein antigens of Mycobacterium leprae. Int. J. Lepr. 60(1992)44-53.
10. ILANGUMARAN, S. Profiles of immune responsive tibody for the serology of leprosy. Clin. Exp. Immunol. 62(1985)468-473.
11. IVANYI, J., SHARP, K., JARKETT, P. and BOTHAMLEY,G. Immunological study of the defined constituents of mycobacteria. Springer Semin. Immunopathol. 10(1988)279-300.
12. JINDAL, S., DUDANI, A. K., SINGH, B., HARLEY, C.B. and GUPTA, R. S. Primary structure of a,human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol. Cell. Biol. 9(1989)2279-2283.
13. KHAN, M. B., DESHPANDE, R. G., DAVIDSON, S. K.and NAVALKAR, R. J. Sero-immunoreactivity of cloned protein antigens of Mycobacterium leprae. Int. J. Lepr. 60(1992)195-200.
14. KLATSER, P. R., DE WIT, M. Y. and KOLK, A. H.An ELISA- inhibition test using monoclonal antibody for the serology of leprosy. Clin. Exp. Immunol. 62(1985)468-473.
15. KORTHAUER, U., GRAF, D., MAGES, H. W., BRIERE,F., PADAYACHEE, M., MALCOM, S., UGAZIO, A. G.,NOTARANGELO, L. D., LEVINSKI, R. J. and KROCZEK, R. A. Defective expression of T-cell CD40 ligandcauses X-linked immunodeficiency with hyper-IgM. Nature 361(1993)539-541.
16. LEVIS, W. R., MEEKER, H. C, SCHULLER-LEVIS, G. B., GILLIS, T. P., MARINO, L. J. JR. and JABRISKIE, J. Serodiagnosis of leprosy: relationships between antibodies to Mycobacterium leprae phenolic glycolipid I and protein antigens. J. Clin. Microbiol. 24(1986)917-921.
17. MATHEW, J. M. and MUTHUKKARUPPAN, V . Class specific immunoglobulins and antibodies to mycobacterial sonicates and autoantigens in leprosy patients and contacts. Microbial Pathol. (1993) (in press).
18. MEEKER, H. C, WILLIAMS, D. L., ANDERSON, D. C, GILLIS, T. P., SCHULLER-LEVIS, G. and LEVIS, W. R. Analysis of human antibody epitopes on the 65-kilodalton protein of Mycobacterium leprae by using synthetic peptides. Infect. Immun. 57(1989)3689-3694.
19. RIDLEY, D. S. and JOPLING, W . H. Classification of leprosy according to immunity-a live-group system. Int. J. Lepr. 34(1966)255-273.
20. ROCHE, P. W. , PRESTIDGE, R. L., WATSON, J. D. and BRITTON, W . Antibody responses to the 18kDa protein of Mycobacterium leprae in leprosy and tuberculoid patients. Int. J. Lepr. 60(1992)201-207.
21. SENGUFIA, U . Does serology help in diagnosing early leprosy? Indian J. Lepr. 65(1993)39-48.
22. SHINNICK, T. M., SWEETSER. D., THOLE, J., VAN EMBDEN, J. and YOUNG, R. A. The etiologic agents of leprosy and tuberculosis share an immunorcactive protein antigen with the vaccine strain Mycobacterium bovis BCG. Infect. Immun. 55(1987) 1932-1935.
23. SIBLEY, L. D., HUNTER, S. W., BRENNAN, P. J. and KRAHENBUHL, J. L. Mycobacterial lipoarabino-mannan inhibits gamma interferon-mediated activation of macrophages. Infect. Immun. 56(1988)1232-1236.
24. SINHA, S., MCENTEGART, A., GRIDHAR, B. K.,BHATIA, A. S. and SENGUPTA, U. Appraisal of two Mycobacterium leprae -specific serological assaysfor monitoring chemotherapy in lepromatous (LL/BL) leprosy patients. Int. J. Lepr. 57(1989)24-32.
25. SINHA, S., SENGUPTA, U., RAMU , G. and IVANYI, J. A serological test for leprosy based on competitive inhibition of monoclonal antibody binding to the My2a determinant of M. leprae. Trans. R. Soc. Trop. Med. Hyg. 77(1983) 869-871.
26. SMITH, P . G. The serodiagnosis of leprosy. Lepr. Rev. 63(1992)97-100.
27. THOLE, J. E. R. and VAN DER ZEE, R. The 65kDa antigen: molecular studies on a ubiquitous antigen. In: Molecular Biology of Mycobacteria. McFadden, J., ed. London: University of Surrey Press, 1990, pp. 37-68.
28. VADIEE, A. R., GILLIS, T. P. and SHANNON, E. J. Confirmation of a false-positive result associated with a competition inhibition assay used for detecting antibodies to a protein epitope of Mycobacterium leprae. Clin. Exp. Immunol. 79(1990)397-402.
29. WATSON, J. D. Leprosy: understanding protective immunity. Immunol. Today 10(1989)218-221.
30. YAMAMURA, M., UYEMURA, K., DANS, R. J., WEINBERG, K., REA, T. H., BLOOM, B. R. and MODLIN, R. L. Defining protective responses to pathogens: cytokine prolifes in leprosy skin lesions. Science 254(1991)277-279.
31. YOUNG, D., LATHIGRA, R., HENDRIX, D., SWEET-SER, D. and YOUNG, R. Stress proteins are immune targets in leprosy and tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 85(1988)4267-4270.
32. YOUNG, D. B. and BUCHANAN, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
33. YOUNG, D. B., KAUFMANN, S. H. E., HERMANS, P. W. M. and THOLE, J. E. R. Mycobacterial antigens: a compilation. Mol. Microbiol. 6( 1992)133-145.
34. YOUNG, R. A., MEHRA, V., SWEETSER, D., BUCHANAN, T. M., CLARK-CURTISS, J., DAVIS, R. W. and BLOOM, B. R. Genes for the major protein antigens of Mycobacterium leprae. Nature 316(1985)450-452.
1. M.Sc; Department of Immunology, School of Biological Sciences, Madurai Kamaraj University, Madurai 625021, India.
2. Ph.D., Department of Immunology, School of Biological Sciences, Madurai Kamaraj University, Madurai 625021, India.
3. M.B.B.S., D.T.M.&H.; Voluntary Health Services, Leprosy Project, Sakthinagar 638315, India.
4. M.D., Voluntary Health Services, Leprosy Project, Sakthinagar 638315, India.
Reprint requests to Dr. Muthukkaruppan.
Received for publication on 10 August 1993.
Accepted for publication in revised form on 5 January 1994.