- Volume 62 , Number 2
- Page: 263–37
An evaluation of the S-100 stain in the histological diagnosis of tuberculoid leprosy and other granulomatous dermatoses
ABSTRACT
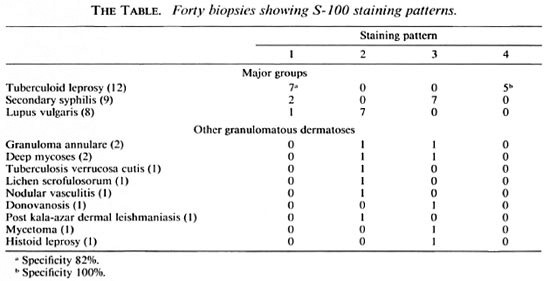
Forty biopsies of granulomatous dermatoses, 12 of which were tuberculoid leprosy (TL), were studied for patterns of nerve twig distribution using an immunoperoxidase technique for S-100 protein. Four distinct patterns of nerve twigs were identified: 1) within granulomas, 2) between granulomas, 3) within and between granulomas, and 4) undetectable nerve twigs in an adequate biopsy. Pattern 4 was seen exclusively in TL (p < 0.05). The other patterns occurred in nonleprosy dermatoses as well, suggesting that pattern 4 is the best indicator toward a diagnosis of TL. The granules of mycetoma and Mycobacterium leprae also stained positively with the S-100 stain.RÉSUMÉ
Quarante biopsies de dermatoses granulomateuses, parmi lesquelles 12 de lèpre tuberculoide (LT), ont été étudiées quant à leurs modes de distribution en filets des nerfs par une technique d'immunoperoxydase pour la protéine S-100. Quatre types distincts de filets nerveux ont été identifiés: 1) dans les granulomes, 2) entre les granulomes, 3) dans et entre les granulomes, et 4) des filets nerveux indétectables dans une biopsie adéquate. Le type 4 était vu exclusivement dans la lèpre tuberculoide (p < 0.05). Les autres types survenaient également dans les dermatoses non-l'éprcuscs, suggérant que le type 4 est le meilleur indicateur pour un diagnostic de lèpre tuberculoide. Les granules de mycétomes et de Mycobacterium leprae prenaient également la coloration au S-100.RESUMEN
Utilizando un técnica de inniunodelección con peroxidasa para la proteína S-100, se estudiaron 40 biopsias de dermatosis granulomatosas (12 de las cuales fueron de lepra tuberculoide, TL) para establecer el patrón de distribución de las ramificaciones nerviosas. Se identificaron 4 patrones distintos de distribución de las ramificaciones nerviosas: (1) dentro de los granulomas, (2) entre los granulomas, (3) dentro y entre los granulomas, y (4) ausencia de ramificaciones nerviosas aún cuando la biopsia fue adecuada. El patrón 4 se observó exclusivamente en los pacientes TL (p menor de 0.05). Ya que los otros patrones se observaron también en dermatosis no leprosas, se sugiere que el patrón 4 es el mejor indicador para establecer un diagnóstico de TL. Los granulos de micetoma y Mycobacterium leprae también se tiñeron positivamente con la tinción para S-100.Leprosy is the most frequently biopsied dermatologic condition in our hospital and contributes approximately 60% of all skin biopsies (290 of 467 in 1991). The majority of leprosy patients attending our outpatient department have tuberculoid leprosy (TL) (BT leprosy in the Ridley-Jopling classification). A search for Mycobacterium leprae in tissue sections stained with the Fite stain (1) is frequently futile. In such a situation we base the diagnosis of TL on a combination of physical signs (12) and histological findings (9). We feel that in an endemic area a diagnosis of leprosy should be entertained in all cases with a compatible clinical presentation and a histologic picture of compact epithelioid cell dermal granulomas, even in the absence of demonstrable M. leprae.
As with other workers (8,11) there have been instances when we have had to revise our initial clinical and histopathological diagnosis of tuberculoid leprosy. In our experience some of the lesions simulating TL include granulomatous secondary syphilis, post kala-azar dermal leishmaniasis, sarcoidosis, granuloma annulare, and lupus vulgaris.
Recent reports in the literature (2,3) have addressed the utility of the immunoperoxidase staining of S-100 proteins in skin biopsies to aid in the diagnosis of tuberculoid leprosy. The positive staining of Schwann cells allows easy recognition of intact dermal nerve twigs or their fragments. It has been proposed that the presence of intact nerve twigs, or fragmented remnants of nerves within the granulomas, should favor a diagnosis of TL (3); the nerves, being protected sites for the location of leprosy bacilli, are the seat of the granuloma formation.
We therefore decided to test the applicability of these findings, using the S-100 stain, in differentiating TL from the other granulomatous conditions that we commonly encounter.
MATERIALS AND METHODS
Twelve consecutive skin biopsies from patients with clinical diagnoses of BT leprosy, based on the findings of hypopigmented, anesthetic lesions, characteristic of the disease in this part of the spectrum (as defined by Ridley and Jopling), were taken for the study. The essential histologic picture in all of these cases was that of a dermal granulomatous inflammation with destruction of the appendages. M. leprae could not be found using the Fite stain.
For comparison, all the "nonleprosy" granulomatous dermatoses (28 cases) encountered in the 3-year period between January 1989 and December 1991 were taken for study (The Table). The two major groups with histology closely resembling that of tuberculoid leprosy were granulomatous secondary' syphilis (SS) and lupus vulgaris (LV). The remainder was comprised of infrequently occurring granulomatous conditions with tissue reactions varying from epithelioid cell granulomas (lichen scrofulosorum) to histiocytic granulomas (histoid leprosy), mixed cell granuloma (fungal) and palisading granulomas (granuloma annulare). In all of the cases studied the clinical and histologic criteria were sufficiently characteristic of the diagnosis.
Formalin-fixed, routinely processed, paraffin-embedded tissue was cut at 4 to 6 /urn. Sections were stained by hematoxylin and cosin (H&E) and a commercially available kit (DAKOPATTS, K524) for demonstration of S-100a antigen by the peroxidaseantiperoxidase (PAP) method (4). This identifies nerves in tissue sections. Statistical analysis of the three major groups was done using Fisher's exact test.
RESULTS
Fibrillar structures staining positively for S-100 protein were identified as nerve twigs. The smallest twigs were approximately 30-µm in size. Four patterns of nerve twig distribution were seen: 1) nerve twigs within granulomas (Fig. 1), 2) nerve twigs between granulomas, 3) nerve twigs within and between granulomas (Fig. 2), and 4) undetectable nerve twigs in an adequate biopsy which included some subcutaneous tissue. In all 12 of our cases of tuberculoid leprosy (The Table) nerve twigs were detectable either within the granulomas (7) or not at all (5). The complete absence of nerve twigs (pattern 4) in an adequate biopsy is the most reliable criterion for the diagnosis of TL (p < 0.05). In the seven cases of TL with nerve twigs present within granulomas, nerve twigs invariably were absent between them (pattern 1). A pattern similar to this also was observed in two cases of SS and one of LV. In LV and SS the dominant patterns were 2 and 3, respectively.
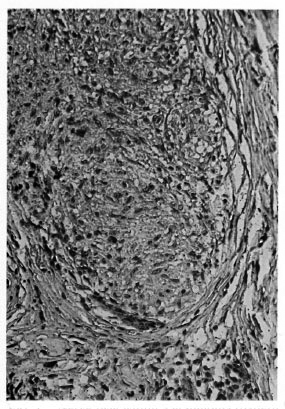
Fig. 1. Nerve twig within a granuloma (pattern 1) detected by S-100 protein in a case of lupus vulgaris (PAP x400).
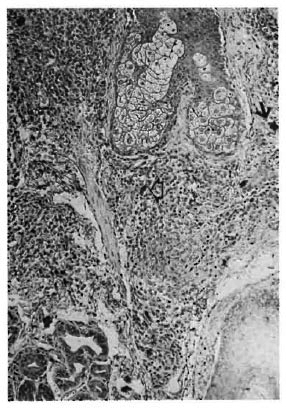
Fig. 2. Nerve twigs both within (open arrowhead) and between (arrow) granulomas (pattern 3) in a case of granulomatous secondary syphilis (PAP x400).
In the remaining cases nerve twig distribution patterns were either 2 or 3. However, the numbers were insignificant for characterizing any pattern of distribution. An additional finding was the positive staining of M. leprae (Fig. 3) in nodular leprosy as well as the granules of mycetoma (Fig. 4) in one case each.
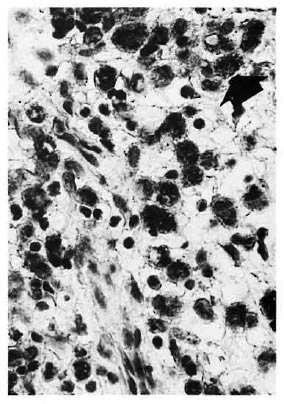
Fig. 3. Numerous macrophages packed with positively staining M. leprae (arrow) (PAP x400).
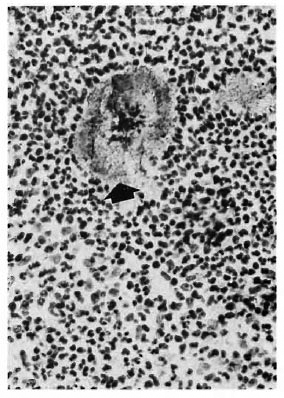
Fig. 4. Positively staining mycetoma granule (arrow) (PAP x400).
DISCUSSION
An etiological diagnosis of epithelioid granulomas of the skin is often difficult because it is seldom possible to demonstrate the inciting agent. Also, different etiological agents produce a confusingly similar morphological picture. Demonstrable evidence of nerve involvement by the granulomatous infiltrate has been considered an important feature of tuberculoid leprosy, its significance being almost the same as the finding of acid-fast bacilli in a nerve (l0). The difficulty in recognizing small nerve twigs on routine H&E sections has led to the use of the S-100 stain as an aid to the histological differentiation of granulomatous skin inflammations.
It has been advocated that the finding of nerve twigs within a granuloma should suggest a diagnosis of leprosy (2,3). This study suggests that such an hypothesis might be misleading because nerves within granulomas also were found in other conditions, notably lupus vulgaris (1 of 7) and granulomatous secondary syphilis (2 of 9). Moreover, even with use of the S-100 stain we were unable to distinguish between the morphology of intact small nerve twigs and fragments of destroyed nerves in the tissue sections, the smallest structures recognizable as nerves consisting of at least 2 to 3 positively staining, fibrillar Schwann cells of approximately 30 µm in size.
It is evident from The Table that two patterns of staining, the presence of nerve twigs within granulomas (pattern 1) or the complete absence of recognizable nerve twigs (pattern 4) in the biopsy, were seen in TL. Although in the majority of the other granulomatous lesions studied the patterns varied, a striking common histological feature was perineural lymphocytic infiltrate, sometimes infiltrating the perineurium. Careful neurological testing in nonleprosy granulomatous dermatoses is likely to reveal clinical evidence of nerve damage as described in cutaneous leishmaniasis (5) and necrobiosis lipoidica (7).
In conclusion, our findings in granulomatous dermatoses suggest that, contrary to the existing view, it is the complete absence of nerve twigs (pattern 4) in an adequate skin biopsy that is the most reliable criterion for the diagnosis of TL. The finding of nerve twigs within but not between granulomas (pattern 1) is highly suggestive of leprosy. The other two patterns, nerve twigs both within and between (pattern 3) or only between granulomas (pattern 2) should suggest diagnoses other than TL. An unexpected finding of this study is the positive staining of the granules of mycetoma. Also, we substantiate a previous report of the strongly positive staining of M. leprae (6) in nodular leprosy.
REFERENCES
1. FITE, G. L., CAMBRE, P. J. and TURNER, M. H. Procedure for demonstrating lepra bacilli in paraffin sections. Arch. Pathol. 43(1947)624-625.
2. FLEURY, R. N. and BACCHI, C . E. S-100 protein and immunoperoxidase technique as an aid in the histopathologic diagnosis of leprosy. Int. J. Lepr. 55(1987)338-344.
3. JOB, C. K., DRAIN, V., DEMING, A. T., HASTINGS, R. C. and GERBER, M . A. Role of S-100 protein as a marker for Schwann cells in the diagnosis of tuberculoid leprosy. (Letter) Int. J. Lepr. 58(1990)392-393.
4. KAHN, H. J., MARKS, A., THOM, H. and BAUMAI, R. Role of antibody to S-100 protein in diagnostic pathology. Am. J. Clin. Pathol. 79(1983)341-347.
5. KUBBA, R., EL-HASSAN, A. M., AL-GINDAN, Y., OMER, A. H., BUSRA, M. and KurrY, M. K. Peripheral nerve involvement in cutaneous leishmaniasis (Old World). Int. J. Dermatol. 26(1987)527-531.
6. LEFFORD, M. J. S-I00 protein marker. (Letter) Int.J. Lepr. 59(1991)122.
7. MANN, R. J. and HARMAN, R. M. Cutaneous anesthesia in necrobiosis lipoidica. Br. J. Dermatol.110(1984)323-325.
8. PAVITHRAN, K. Chromoblastomycosis masquerading as tuberculoid leprosy. (Letter) Int. J. Lepr.60(1992)657-658.
9. RIDLEY, D. S. Histological classification and immunological spectrum of leprosy. Bull. WHO 51(1974)451-465.
10. RIDLEY, D. S. Skin Biopsy in Leprosy . 2nd edn. Basle: CIBA-GEIGY, 1985, p. 30.
11. SINGH, K., DAWAR, R. and RAMESH, V. Lymphocytic infiltration of skin of Jessner-Kanof masquerading as borderline leprosy. Indian J. Lepr.57(1985)804-806.
12. WORLD HEALTH ORGANIZATION. A Guide to Leprosy Control. 2nd edn. Geneva: World Health Organization, 1998.
1. M.D., Lecturer, Department of Pathology, University College of Medical Sciences and GTB Hospital, Delhi 110095, India.
2. M.D., Reader; Department of Pathology, University College of Medical Sciences, Delhi 110095, India.
3. M.D., Lecturer; Department of Pathology, University College of Medical Sciences, Delhi 110095, India.
4. M.D., Professor, Department of Pathology, University College of Medical Sciences, Delhi 110095, India.
5. M.D., Assistant Professor, Department of Dermatology, All India Institute of Medical Sciences, New Delhi 110029, India.
Received for publication on 6 July 1993.
Accepted for publication in revised form on 1 March 1994.