- Volume 62 , Number 2
- Page: 268–77
Progress in the chemotherapy of leprosy*
Editorial opinions expressed are those of the writers.
We are pleased to have the opportunity of publishing the full texts of the stateof-the-art lectures presented at the XIV International Leprosy Congress in Orlando, Florida, U.S.A., 29 August-4 September 1993. The first two of these lectures appear in the March 1994 issue. Remaining lectures will appear in subsequent issues.- RCH
Although they are a minority in number, patients with lepromatous or multibacillary leprosy are the main sources of infection with Mycobacterium leprae and those in whom drug-resistant mutants are the most easily selected. The rationale that supports modern chemotherapy for leprosy (6,7,15,24,42) is the same as that of chemotherapy for patients with cavitary pulmonary tuberculosis. In both cases, the multibacillary character of the disease is the crucial point.
MICROBIAL POPULATION IN MULTIBACILLARY LEPROSY
In a patient with multibacillary (MB) leprosy the maximal number of acid-fast bacilli (AFB) could be 100 billion (1011) or 1 trillion (1012), of which on average only 1% (109 or 1010) are viable (36). The proportion of M. leprae mutants resistant to dapsone, clofazimine or rifampin among those 109 - 1010 viables has not yet been established because until now M. leprae cannot be cultivated in vitro. However, by analogy with what is known about M. tuberculosis (3.4.16.27) the mean proportion of drug-resistant mutants within a wild strain of M. leprae was estimated to be 1 per 10 million, 10 -7 to rifampin and 10-6 to dapsone, clofazimine or thioamide, cither ethionamide or prothionamide. Thus, a patient with MB leprosy harbors, at most, 10'' drug-susceptible organisms, 103 rifampin-resistant mutants, and 104 dapsone-, clofazimine or thioamide resistant mutants (Fig. 1).
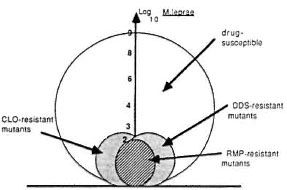
Fig. 1. Bacillary populations in multibacillary leprosy.
BASIS OF CHEMOTHERAPY FOR LEPROSY
In order to cure a patient microbiologically, chemotherapy should be capable of killing all viable organisms, the drug-resistant mutants as well as the drug-susceptible organisms. Killing the drug-susceptible organisms requires a drug or a combination of drugs with bactericidal activities. The more bactericidal against all forms of drugsusceptible organisms the prescribed drug(s), the shorter the total duration of treatment and the more effective the chemotherapy, the smaller the percentage of relapses with drug-susceptible organisms after stopping treatment. It should be clearly understood therefore that the length of treatment is highly dependent upon the capability of the drug therapy to sterilize the lesions. Killing the drug-resistant mutants requires a more sophisticated approach. It requires the use of a combination of drugs to kill mutants resistant to each drug in order to prevent the selection of resistant mutants that would lead to treatment failure. The killing of drug-resistant mutants and the prevention of their selection is, therefore, the first objective of chemotherapy.
Killing drug-resistant mutants To kill resistant mutants one takes advantage of a basic rule of genetics, the independence of mutation. According to that rule, a mutant resistant to a given drug remains fully susceptible to another drug on the condition that the latter has a mechanism of action different from that of the former. In other words, each drug acts against the mutants resistant to the others. Because of the relatively limited size of the drug-resistant mutants in multibacillary leprosy before treatment, the combination of two drugs, for example, rifampin and dapsone, is in theory sufficient to kill the resistant mutants (Table 1). However, one should keep in mind the possibility of primary and secondary dapsone resistance. In practice, to overcome possible dapsone resistance the recommendation is to combine three drugs, rifampin, dapsone and clofazimine (42) (Fig. 2).
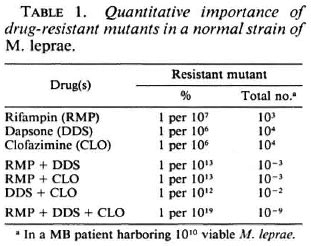
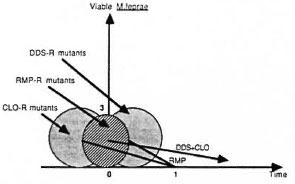
Fig. 2. Outcome of drug-resistant mutants in patients on combined chemotherapy (MDT).
Dapsone, being poorly bactericidal, has to be given at a daily dose of 100 mg in adults. Clofazimine, also poorly bactericidal, has to be given daily at a dose of 50 mg with a monthly supplement of 300 mg, but might be given monthly at a dose of 1200 mg (23). For rifampin, a single dose of 600 mg kills more than 99% of the susceptible organisms present at the start of treatment, that is as much as 3 to 6 months of daily treatment with the combination dapsone plus clofazimine (7,28,37,39) Since daily treatment with rifampin did not demonstrate to be more bactericidal than monthly treatment (39), there is no need to give rifampin daily, even during the initial phase of chemotherapy. By definition, mutants resistant to dapsone and clofazimine are fully susceptible to rifampin and are killed by the first dose(s) of rifampin; whereas it takes a much longer duration of daily administration of dapsone and clofazimine to get rid of rifampin-resistant mutants. This explains why in the control programs it is recommended to give only monthly supervised pulses of 600 mg rifampin, a rhythm of administration that fits well with the regular field visits of the medical team. On the other hand, since the length of time needed to kill rifampin-resistant mutants cannot be determined precisely, in order to remain on the safe side it is recommended to administer the combination dapsone-clofazimine during the whole course of therapy.
Killing drug-susceptible organisms
During the initial phase of MDT, in addition to the killing of drug-resistant mutants there is also an extremely rapid killing of drug-susceptible organisms (Fig. 3). For example, in patients of the Bamako-Chingleput study (39), the proportion of M. leprae remaining capable of multiplying in the foot pads of mice after the few initial doses of 600 mg rifampin was in the range of 10-7, i.e., 1 per 10,000,000. Since about 10-2 (1 per 100) were able to multiply before treatment, then the first doses of rifampin have reduced the proportion of viables from 10-2 to 10-7 , in other words have killed 99.999% of them. The initial population of viable M. leprae in a MB patient has therefore been reduced from 1010 to 105. The objective is then to eliminate the remaining 105 viable drug-susceptible M. leprae in order to prevent relapse after stopping treatment.
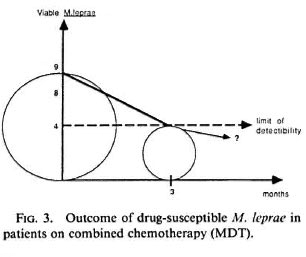
Fig. 3. Outcome of drug-susceptible M. LEPRAE in patients on combined chemotherapy (MDT).
After the initial rapid killing of M. leprae in the Bamako-Chingleput study (39), the proportion of M. leprae capable of multiplying in mice remained constant during the 2 years of treatment, suggesting that chemotherapy was mostly ineffective against the remaining organisms. These organisms were not drug-resistant M. leprae selected by the chemotherapy because the few bacilli that grew in the foot pads of mice were normally susceptible to rifampin and other drugs. They are unresponsive to the drugs to which they are fully susceptible, perhaps because of the strong post-antibiotic effect (31)of rifampin. The suppression of metabolic activity, that persists after short exposure of organisms to antimicrobials and might be due to the nonlethal damage caused by antimicrobials, has been observed in leprosy after long-term dapsone therapy and in many other infectious diseases including tuberculosis (30,40) and staphylococcus infection.
Faced with the apparent secondary unresponsiveness of M. leprae to chemotherapy after the initial doses of rifampin, the crucial question is whether or not it is worth it to continue the treatment, in other words, what is the optimal duration of chemotherapy? There is no definite answer to that question but only some suggestion that it is useful to continue treatment beyond the first doses of rifampin. For example, in a recent study of MB patients who relapsed after very short-course MDT (29) it was clear that the shorter the duration of treatment with rifampin, the shorter the incubation period of relapse (Table 2).
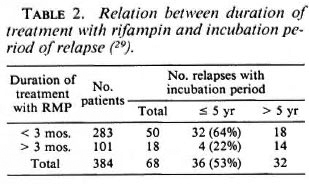
There is, therefore, a contradiction between the apparent unresponsiveness of a tiny proportion of M. leprae to chemotherapy and the apparent benefit of prolonging chemotherapy. A hypothesis to reconcile these apparently opposite facts would be that the role of drug therapy, after initial doses of rifampin that have killed 99.999% of the bacillary population, is to maintain the remaining organisms in their dormant state so that the clearing mechanisms of the host can eliminate them progressively. If such a hypothesis was valid then it might be possible to determine the optimal length of treatment. In the Bamako-Chingleput study, the load of acid-fast bacilli (AFB) in the serial skin smears decreased by 0.62 log1 0 per year. Because the proportion of viable
M. leprae among AFB remained constant (10-7) for the 2 years of the study, then the clearing of the remaining viable M. leprae should be parallel to the clearing of AFB in the skin smears, also 0.62 log10 per year. The time required to clear 105 M. leprae at a speed of 0.62 log10per year is about 7-8 years for a MB case of leprosy with maximal bacterial load (Fig. 4). This is more or less the time necessary for a MB patient with a bacterial index (BI) of 4 to 5 + to reach skinsmear negativity. Of course, for MB patients with a lower initial BI, the optimal length of chemotherapy would depend upon the initial load of AFB. For previously dapsonetreated patients with already negative skin smears, the optimal length of combined chemotherapy could well be still shorter.
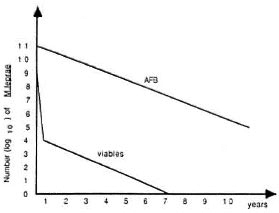
Fig. 4. Possible outcome of M. leprae AFB and viables in a multibacillary patient on combined chemotherapy (MDT).
A recent finding of the Marchoux Chemotherapy Study Group (29) gives an indirect support to the hypothesis of a relation between bacterial load and optimal length of treatment. As shown in Table 3, relapses after very short-course MDT were significantly earlier and more numerous among MB patients whose BI upon completion of treatment was 5 + or more than among those with a BI <5 + , suggesting that the higher the BI the longer the required length of treatment.
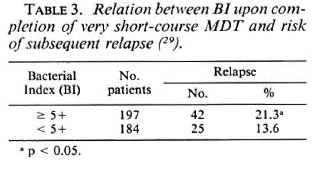
In the control programs it is difficult to adapt the length of treatment to the bacterial load for each patient. Operationally it is easier to standardize the duration of treatment for the two main categories of patients, those whose BI is negative and considered as paucibacillary and those whose BI is positive and considered multibacillary. According to the recommendations of the Sixth Report of the Expert Committee on Leprosy (41), the former should treated for months whereas the latter should be treated for at least 2 years and at best until smear negativity. To prolong treatment up to smear negativity might be especially beneficial for the most bacillary cases of leprosy because those cases have the lowest specific immunity against leprosy and have likely the least capacity to maintain the remaining M. leprae in a dormant state.
PRESENT RESULTS OF COMBINED CHEMOTHERAPY (MDT) FOR LEPROSY
Since the WHO recommendations (41,42) multidrug therapy (MDT) has become routine practice in a majority of endemic countries. By the middle of 1993, about 4.2 million patients have been treated with success. Clinical improvement has been rapid, side effects rare and mild, and compliance satisfactory. The mean cumulative risk of relapse has been 1% over 9 years of follow up (S. K. Noordeen, personal communication, 1993).
Among PB cases of leprosy, clinically active skin lesions were still present at the end of the 6-month course of MDT among 4% to 28% of the patients (2,26). For patients and health workers, these lesions were still considered as active and requiring treatment even though they usually improved or disappeared after stopping MDT (1). In addition, reversal reactions may happen after stopping treatment (2,26) and cause nerve damage. Because of the relatively low frequencies of these findings, it is not recommended to extend the 6-month duration of treatment for PB leprosy up to 12 months. Nevertheless, prolongation or réintroduction of MDT may be considered on an individual basis (41).
Among MB cases of leprosy, less than 1% of relapses have been observed within the first 5 years of follow up after completion of treatment in field programs as well as in field trials conducted by WHO, and even among newly diagnosed patients who received only 2 years of MDT. Such a finding is strong evidence of the potent bactericidal activity of the recommended regimen, and of its capacity to cope with persisters. Another finding of crucial importance is that, among MB patients who relapsed after an extremely short course of MDT containing rifampin(29), and in the single patient who relapsed after 2-year daily MDT in Martinique (5) whose M. leprae strains were isolated on mice and their drug susceptibility tested, all strains remained fully susceptible to the prescribed drugs. MDT is, therefore, capable of reaching its first objective, the killing of drug-resistant mutants.
RESEARCH IN CHEMOTHERAPY OF LEPROSY
Because of the excellent activity of the WHO-recommendation regimens, the priority in the chemotherapy for leprosy is to implement MDT as rapidly and as extensively as possible in all endemic countries. It is also important to conduct operational research and health system research, aiming to improve the average of MDT implementation, and assessing its results in terms of compliance, completion and epidemiologic impact.
Meanwhile, research in chemotherapy is conducted with the following two major objectives: to shorten the duration of the present regimens and to improve their supervision. Compared to life-long dapsonc monotherapy, the 2-year MDT for MB leprosy has made considerable progress but it will take a long time and will be difficult to implement in some areas (24), and in the areas where it is implemented the patients' compliance is often questionable (8). In addition, the classification of patients into PB and MB leprosy requires microscopic examination of skin smears. To have a shorter and/or fully supervised treatment applicable to both PB and MB patients would, therefore, represent great progress.
With the presently available drugs (rifampin, dapsonc and clofazimine) there is not much hope to shorten the overall duration of MDT because only rifampin has very strong bactericidal activity. It is not possible to strengthen MDT activity by adding a thioamide because thioamides are poorly bactericidal drugs and have the potential for liver toxicity. On the other hand, compliance with MDT might be improved, for example, by giving only monthly supervised doses of clofazimine. The recent study by Jamet, el al. (23), demonstrating that clofazimine was as bactericidal at 1200-mg monthly doses as at 50-mg daily doses combined with a 300-mg monthly dose, is encouraging in that respect.
New drugs with bactericidal activity against M. leprae
Actually to shorten treatment duration and develop fully supervised intermittent regimens, new drugs with strong bactericidal activity against M. leprae are needed. Such drugs are presently available, namely, fluoroquinolones, minocycline and clarithromycin. All of them are lipophilic drugs, thus capable of penetrating eukaryotic cell membranes and the cell wall of mycobacteria.
Fluoroquinolones. Fluoroquinolones, nalidixic acid derivatives, inhibit bacterial DNA gyrase, an enzyme which is involved in DNA replication. They have a mechanism of action totally different from that of other antileprosy drugs. Among them, pefloxacin and ofloxacin have a powerful bactericidal activity against M. leprae in the mouse model (10,17,20,33,35) hown in Table 4, 22 daily doses of pefloxacin 800 mg or ofloxacin 400 mg are killing 99.99% of viable M. leprae present in MB leprosy at the start of treatment (19,32). Definite clinical improvement was observed in all patients, and the morphological index (MI) drastically decreased to the baseline during treatment. Increasing the daily dose of ofloxacin up to 800 mg did not increase the bactericidal activity, and the combination with dapsone and clofazimine did not enhance or reduce its activity (Ji, et al, submitted).
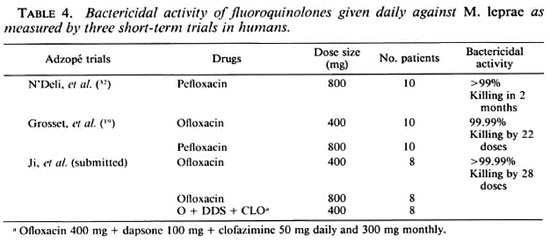
It is important to emphasize that, except for rifampin, no other drug so far tested in humans has demonstrated such a degree of bactericidal activity. Since daily ofloxacin for 4 weeks is capable of killing 99.99% or 4 log10 M. leprae, i.e., as many organisms as the maximal number of rifampin-resistant mutants in a MB patient, then it is possible that the daily combination of ofloxacin and rifampin may permit us to shorten considerably the duration of MDT. A multicenter field trial is being organized under the auspices of WHO to test this hypothesis.
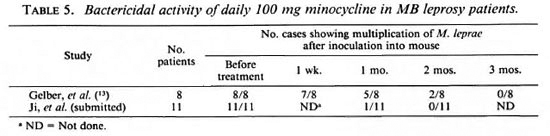
Minocycline. Among the tetracyclines, minocycline is unique in being active against M. leprae (11,12). It acts in inhibiting protein synthesis by a mechanism different from that of other antileprosy drugs. In mice, its bactericidal activity is similar to that of ofloxacin (11,25). In humans given a daily dose of 100 mg minocycline (13 and Ji, et al, submitted), definite clinical improvement was seen in some patients as early as 14 days after beginning treatment, in all patients by 1 month, and marked improvement in all patients by 2 months. As also observed with fluoroquinolone treatment, the change in the MI was spectacular. Table 5 summarizes the results of mouse subinoculations from serial skin samples, showing that clearance of viable M. leprae was rapid. More than 99% and 99.9% of the viable M. leprae had been killed by 28 and 56 days of treatment, respectively, a result similar to that obtained with ofloxacin.
Clarithromycin. A derivative of erythromycin, clarithromycin is a new macrolide that inhibits protein synthesis by a mechanism different from that of minocycline. In mice, its bactericidal activity is of the same order as that of ofloxacin and minocycline (9,25). In MB leprosy patients treated daily with 500 mg clarithromycin, the clinical response, the MI decrease and the killing of viable M. leprae are also of the same order as that of ofloxacin and minocycline (Ji, et ai, submitted). Although some synergism between minocycline and clarithromycin was demonstrated in the mouse model, the combination of 100 mg minocycline and 500 mg clarithromycin was not more active in man than each component alone, perhaps because the potency of each individual drug was too strong.
Research for new drug regimens
New drug regimens for leprosy have potential interest only if they are better than the WHO-recommended regimens in terms of antimicrobial activity, acceptability and safety, overall cost-effectiveness, length of treatment, compliance and/or if they may replace them in case of proven resistance to rifampin.
It is clear that the three new drugs ofloxacin, minocycline and clarithromycin are individually and in combination much more active than dapsone and clofazimine. They are, therefore, natural candidates to replace dapsone and clofazimine for improving leprosy chemotherapy. Among numerous possible improvements, the following might be considered.
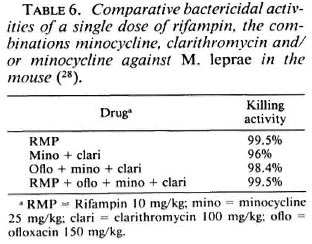
Improved supervision of present MD T regimens. A disadvantage of the presently recommended MDT regimens in that only the monthly doses of 600 mg rifampin and 300 mg clofazimine are supervised. If a patient is not at all compliant in the intake of daily unsupervised doses of dapsone and clofazimine, then his treatment actually might be in practice monotherapy by rifampin, with the risk of developing secondary resistance to rifampin. A possibility, already discussed, is to administer clofazimine monthly at a dose of 1200 mg. But the tolerance of such a high dose of clofazimine is questionable in the long term. Another, more tempting, possibility is to administer monthly doses of a combination of the new drugs ofloxacin, minocycline and clarithromycin. A single dose of ofloxacin alone did not demonstrate sufficient bactericidal activity (19) to be recommended. On the other hand, as shown in Table 6, a single dose of either the double-drug combination (minocycline and clarithromycin) or tripledrug combination (ofloxacin, minocycline and clarithromycin) displays a bactericidal activity close to that of a single dose rifampin in the mouse. In addition, a single dose of the combination of all four drugs is not antagonistic, suggesting that daily dapsone and clofazimine might well be replaced by monthly doses of ofloxacin, minocycline and clarithromycin. The activity of monthly doses of the new drugs given in combination with and without rifampin is presently being tested in the field.
Short-course chemotherapy for leprosy. As already discussed, the daily regimen of 600 mg rifampin and 400 mg ofloxacin for 1 month might be as active as the 2-year drug regimen recommended by WHO. With the former, the patient would receive 28 doses of rifampin and ofloxacin; with the latter the patient would receive 24 doses of rifampin and more than 730 doses of dapsone and clofazimine. Since daily ofloxacin for 1 month is considered as capable as 2 years of dapsone plus clofazimine to kill the rifampin-resistant mutants present at the start of treatment, the patient treated for 1 month with daily rifampin and ofloxacin may well be overtreated compared to the patient treated with the WHO-recommended MDT. To remain still more on the safe side while keeping the advantage of shortcourse chemotherapy, an increase of the bactericidal activity of the drug combined with rifampin has to be considered. Such an increase cannot be obtained by increasing the 400 mg daily dose of ofloxacin to 800 mg, because 800 mg ofloxacin did not demonstrate a better bactericidal activity than 400 mg (Ji, et al., submitted) but might be less well tolerated. On the other hand, it is possible to combine minocycline and clarithromycin with ofloxacin. In spite of the limited sensitivity of the mouse model in measuring strong bactericidal activity, there are some indications that the combination clarithromycin + minocycline (Ji, et al., submitted) and the combination ofloxacin + clarithromycin + minocycline (25) are more powerful than ofloxacin alone (19). These remain to be demonstrated in the field and evaluated in terms of antimicrobial activity, acceptability and tolerance by the patients, and cost effectiveness.
Long-course, fully supervised, intermittent regimens. To successfully treat patients living in remote areas and/or regions where monthly supervised treatment or intensive short-course chemotherapy are difficult to implement, it would be useful to develop new regimens based on rifampin and the newer drugs given with long intermittencies between each pulse of the drugs. In that respect, two findings should be taken into consideration. First, a single pulse of ofloxacin, minocycline plus clarithromycin is as bactericidal as a single pulse of rifampin, thus is capable to kill rifampin-resistant mutants as rapidly as rifampin kills the susceptible organisms. Second, a single pulse of rifampin is capable of rendering for months M. leprae harvested from skin biopsies not cultivable in the mouse. The postantibiotic effect of rifampin, that is due to the persisting damages induced by the drug, is particularly important in the case of M. leprae. Taking advantage of both findings, why not design rifampin-containing regimens including two or three of the newer drugs that would be given under supervision once a month, every 2 months, every 3 months and perhaps every 6 months or even once a year? Of course a number of questions should be answered before introducing such regimens in the field. To the question of the length of drug administration, the answer might be until the skin smear becomes negative. Arguments supporting that answer have been given previously. The question of the prerequisites before testing the regimens in the field might be answered when it has been demonstrated that there is no regrowth of M. leprae within the 3, 6, or even 12 months between each pulse of the drug combinations. Experimental studies presently conducted in nude mice will provide the answer.
Chemotherapy for rifampin-resistant leprosy. From a still limited number of patients (18,22), rifampin-resistant M. leprae have been isolated. Because of their epidemiologic importance, these patients should benefit in priority of the newly developed drugs. After having demonstrated the rifampin-resistance of the organisms, an effective drug regimen (21) for rifampin-resistant leprosy might be as follows. During the first 2 or 3 months, treatment would be daily 400 mg ofloxacin, 500 mg clarithromycin, 100 mg minocycline and 100 mg clofazimine. During the following 6 months treatment would be daily 500 mg clarithromycin, 100 mg minocycline and 50 mg clofazimine and then, up to smear negativity, either 100 mg minocycline and 50 mg clofazimine or 50 mg clofazimine alone, or 100 mg minocycline alone.
- Prof. Jacques-Henri Grosset
Département de Bactériologie- Virologie
Faculté de Médecine Pitie-Salpetriere
91 Blvd. de l'Hôpital
75634 Paris 13, FRANCE
REFERENCES
1. BECX-B LEUMINCK, M. Operational aspects of multidrug therapy. Int. J. Lepr. 57(1989)540-541.
2. BCCRRIGTER, G., PONNIGHANS, S. M. and FINE, P. E. M. Preliminary' appraisal of a WHO-recommended multiple drug regimen in paucibacillary leprosy patients in Malawi. Int. J. Lepr. 56(1988)408-417.
3. CANETTI, G. and GROSSET, J. Teneur des souches sauvages de M. tuberculosis en variants résistants à l'isoniazide et en variants résistants à la streptomycine sur milieu de Lôwenstein-Jensen. Ann. Inst. Pasteur 101(1961)28-46.
4. CANETTI, G., RIST, N. and GROSSET, J. Mesure de la sensibilité du bacille tuberculeux aux drogues antibacillaires par la méthode des proportions. Rev. Tuberc. Pneum. 27(1963)217-272.
5. CONSTANT-D ESPORTES, M., GUELPA-LAURAS, C. G, CAROLINA, J. C, LEOTURE, A., GROSSET, J. and SANSARRICQ, H. A case of relapse with drug-susceptible M. leprae after multidrug therapy. Int. J. Lepr. 59(1991)242-247.
6. ELLARD, G. A. The chemotherapy of leprosy. Part 1. Int. J. Lepr. 58(1990)704-716.
7. ELLARD, G. A. The chemotherapy of leprosy. Part 2. Int. J. Lepr. 59(1991)82-94.
8. ELLARD, G. A., PANNIKAR, V. K., JESUDASAN, K. and CHRISTIAN. M. Clofazimine and dapsone compliance in leprosy. Lepr. Rev. 59(1988)205-213.
9. FRANZBLAU, S. G. and HASTING, R. C. In vitro and in vivo activitiers of macrolides against Mycobacterium leprae. Antimicrob. Agents Chemother. 32(1988)1758-1762.
10. FRANZBLAU, S. G. and WHITE, K. E. Comparative in vilro activities of 20 fluoroquinolones against Mycobacterium leprae. Antimicrob. Agents Chemother. 34(1990)229-231.
11. GELBER, R. H. The use of rodent models in assessing antimicrobial activity against Mycobacterium leprae. Lepr. Rev. 57 Suppl. 3 (1986)137-148.
12. GELBER, R. H. Activity of minocycline in Mycobacterium leprae-infecled mice. J. Infect. Dis. 186(1987)236-239.
13. GELBER, R. H. Activity of minocycline and aminoglycosides for Mycobacterium leprae. Int. J. Lepr. 61(1993)314-315.
14. Global strategy for the elimination of leprosy as a public health problem. Geneva: Leprosy Unit, World Health Organization, 1993.
15. GROSSET, J. H. Recent developments in the field of multidrug therapy and future research in chemotherapy of leprosy. Lepr. Rev. 56 Suppj. (1986)223-234.
16. GROSSET, J. and CANETTI, G. Teneur des souches sauvages de M. tuberculosis en variants résistants aux antibiotiques mineurs (acide para-amino-salicylique, ethionamide, cycloserine, viomycine, kanamycine). Ann. Inst. Pasteur 103(1962)163-184.
17. GROSSET, J. H., GUELPA-L AURAS, C. G, PERANI, E. G. and BEOLETTO, C. Activity of ofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 56(1988)259-264.
18. GROSSET, J. H., GUELPA-LAURAS, C. G, BOBIN, P., BRICKER, G., CARTEL, J. L., CONSTANT-DESPORTES, M., F LAGEUL, B., FRÉDÉRIC, M., GUILLAUME, J. C. and MILLAN, J. Study of 39 documented relapses of multibacillary leprosy after treatment with rifampin. Int. J. Lepr. 57(1989)607-614.
19. GROSSET, J. H., Ji, B., GUELPA-LAURAS, C. G, PERANI, E. G. and N'DELI, L. Clinical trial of pefloxacin and ofloxacin in the treatment of lepromatousleprosy. Int. J. Lepr. 58(1990)281-295.
20. GUELPA-LAURAS, C. G, PERANI, E. G., GIROIR, A. M. and GROSSET, J. H. Activities of pefloxacin and ciprofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 55(1987)70-77.
21. ILEP MEDICAL BULLETIN. Possible chemotherapy of rifampicin resistant leprosy. 1992, Nº 6.
22. JACOBSON, R. R., HASTINGS, R. C. Rifampicinresistant leprosy. Lancet 2(1976)1304-1305.
23. JAMET, P., TRAORE, I., HUSSER, J. A. and Jl, B.Short-term trial of clofazimine in prevously untreated lepromatous leprosy. Int. J. Lepr. 60(1992)542-548.
24. JI, B. and GROSSET, J. Recent advances in the chemotherapy of leprosy. Lepr. Rev. 61(1990)313-329.
25. JI, B., PERANI, E. G. and GROSSET, J. H. Effectiveness of clarithromycin and minocycline alone or in combination against Mycobacterium leprae infection in mice. Antimicrob. Agents Chemother. 35(1991)579-581.
26. KATOCH, K.., RAMANATHAN, U., NATRAJAN, M., BAGGA, A. K., BHATIA, A. S., SAXENA, R. K. and RAMU, G. Relapses in paucibacillary patients after treatment with three short-term regimens containing rifampicin. Int. J. Lepr. 57(1989)458-464.
27. LE LIRZIN, M. and DJUROVIC, V. Etude sur milieu de Lowenstein-Jensen de la composition des souches sauvages de Mycobacterium tuberculosis en variants resistant a la rifampicine et en variants resistant a l'ethambutol. Ann. Inst. Pasteur 120(1971)531-548.
28. LEVY, L., SHEPARD, C. C. and FASAL, P. The bactericidal effect of rifampicin on M. leprae in man: a) single doses of 600, 900 and 1200 mg; and b) daily doses of 300 mg. Int. J. Lepr. 44(1976)183-187.
29. MARCHOUX CHEMOTHERAPY STUDY GROUP. Relapse rates in multibacillary leprosy patients after stopping treatment with rifampin-containing regimens. Int. J. Lepr 60(1992)525-535.
30. McDERMOTT, W. Microbial persistence. Yale J. Biol. Med. 30(1958)257-329.
31. McDONALD, P. J., CRAIG, W. A. and KUNIN, C. M. Persistant effects of antibiotics on Staphylococcus aureus after exposure for limited periods of time. J. Infect. Dis. 135(1977)217-223.
32. N'DELI, L., GUELPA-LAURAS, C. C, PERANI, E. G. and GROSSET, J. H. Effectiveness of pefloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58(1990)12-18.
33. PATTYN, S. R. Activity of ofloxacin and pefloxacin against Mycobacterium leprae in mice. Antimicrob. Agents Chemother. 31(1987)671-672.
34. Progress towards the elimination of leprosy as a public health problem. Weekly Epidemiol. Rec. 25(1993)181-186.
35. SAITO, H., TOMIOKA, H. and NAGASHIMA, K. In vitro and in vivo activities of ofloxacin against Mycobacterium leprae infection induced in mice. Int. J. Lepr. 54 (1986) 560-562.
36. SHEPARD, C. C. Recent developments in the chemotherapy and chemoprophylaxis of leprosy. Leprologia (Argent.) 19 (1974) 230-236.
37.SHEPARD, C. C., LEVY, L. and FASAL, P. Rapid bactericidal effect of rifampicin on Mycobacterium leprae. Am. J. Trop. Med. Hyg. 21 (1972) 446-449.
38.SHEPARD, C. C., LEVY, L. and FASAL, P. Further experience with the rapid bactericidal effect of rifampin on Mycobacterium leprae. Am. J. Trop. Med. Hyg. 23 (1974) 1120-1124.
39. THELEP SUBCOMMITTEE ON CLINICAL TRIALS OF THECHEMOTHERAPY OF LEPROSY (THELEP) SCIENTIF-IC WORKING GROUP OF THE UNDP/WORLD BANK/WHO SPECIAL PROGRAMME FOR RESEARCH ANDTRAINING IN TROPICAL DISEASES. Persisting Mycobacterium leprae among THELEP trial patientsin Bamako and Chingleput. Lepr. Rev. 58 (1987)325-337.
40. WATERS, M. F. R., REES, R. J. W., MCDOUGALL,A. C. and WEDDELL, A. G. M. Ten years of dap-sone in lepromatous leprosy: clinical, bacteriolog-ical and histological assessment and the finding ofviable leprosy bacilli. Lepr. Rev. 45 (1974) 288-298.
41. WHO EXPERT COMMITTEE ON LEPROSY. Sixth Report. Geneva: World Health Organization, 1988.Tech. Rep. Ser. 768.
42. WHO STUDY GROUP. Chemotherapy of leprosy forcontrol programmes. Geneva: World Health Or-ganization, 1982. Tech. Rep. Ser. 675.
* Based on state-of-the-art lecture presented at the XIV International Leprosy Congress on 1 September 1994, Orlando, Florida, U.S.A.