- Volume 62 , Number 2
- Page: 209–14
Relapses after a single dose of rifampin in skin-smear negative multibacillary patients after dapsone monotherapy
ABSTRACT
Between 1982 and 1985, a single 1500 mg dose of rifampin (RMP) was given to 136 multibacillary leprosy patients who had become clinically inactive and skin-smear negative after various durations of dapsone monotherapy, and then antileprosy chemotherapy was totally stopped. By the end of June 1992, 15 relapses were detected among these patients. The overall relapse rate was 11%; the relapse rate per 100 patient-years was 2.1%, which was the highest among those published to date; the cumulative risk of relapse at year 7 of follow up was 8.8%. All of these figures indicate that the relapse rate among this group was at least the same as in other studies where patients received dapsone monotherapy only. Therefore, the administration of a single large dose of RMP could neither prevent relapse nor reduce its rate among multibacillary patients who had already become clinically and skin-smear negative after dapsone monotherapy.RÉSUMÉ
Entre 1982 et 1985, une dose unique de 1500 mg de rifampicine (RMP) fut administrée à 136 patients lépreux multibacillaires qui étaient devenus cliniquement inactifs et avaient des frottis cutanés négatifs après diverses durées de monothérapie à la dapsone. La chimiothérapie anti-lèpre fut ensuite totalement arrêtée. A la fin juin 1992, 15 rechutes avaient été détectées parmi ces patients. Le taux global de rechute était de 11.0%; le taux de rechute par 100 patients-années était de 2.1%, ce qui est le plus élevé publié à ce jour; le taux cumulé de rechute après 7 ans de suivi était de 8.8%. Tous ces chiffres indiquent que le taux de rechute dans ce groupe était au moins équivalent à celui d'autres études où les patients n'avaient reçu qu'une monothérapie à la dapsone. En conséquence, l'administration d'une dose unique élevée de rifampicine n'a pas été capable de prévenir la rechute ni de diminuer son taux chez des patients multibacillaires qui étaient déjà devenus négatifs cliniquement ainsi qu'aux frottis cutanés après une monothérapie à la dapsone.RESUMEN
Entre 1982 y 1985, se administra una sola dosis de 1500 mgde rifampina (RMP) a 136 pacientes con lepra multibacilar que habían llegado a ser clínicamente inactivos y baciloscopicamente negativos como resultado de su tratamiento prolongado con dapsona. Después, el tratamiento antileproso se suspendió totalmente. A finales de 1992 se detectaron 15 pacientes con recaída. La frecuencia global de recaída fue del 11.0%, la tasa de recaída por 100 pacientes/años fue del 2.1% la cual es la más alta publicada hasta ahora; el riesgo acumulativo de recaída a los 7 años de seguimiento fue del 8.8%. Todas estas cifras indican que la frequencia de recaída en este grupo fue al menos igual que la encontrada en otros estudios similares donde los pacientes recibieron solo la monoterapia con dapsona. La administración de una sola dosis de RMP no pudo ni evitar las recaídas ni reducir su frecuencia en una población de pacientes multibacilares que ya habían llegado a ser clínica- y baciloscopicamente negativos después de la monoterapia con dapsona.Relapse in multibacillary (MB) leprosy after dapsone monotherapy has been well documented (1,3,4,10,14,15,20) Patients in these studies were treated either with lifelong dapsone monotherapy (1,4,10,15) or discontinued treatment after various durations of clinical and bacteriological negativity (3,20). The relapse rate ranged from 1.04%(20) to 2.5 (3) per 100 person-years. Although the relapse rate has never been compared between patients who continued and discontinued dapsone monotherapy in a welldesigned clinical trial, it seems unlikely that life-long monotherapy might significantly reduce the relapse rate. It is important to point out that the great majority of patients who relapsed during or after dapsone monotherapy were resistant to dapsone (l6).
Rifampin (RMP) displayed very rapid and powerful bactericidal activity against Mycobacterium leprae in lepromatous leprosy (5.12,17,18) The lost their infectivity to immunocompetent (normal) mice after patients received treatment with single doses of 600, 900 and 1200 mg RMP (12). As in human leprosy, a single dose of 10 mg/ kg RMP also displayed significant bactericidal activity against M. leprae in mice (6-9). Because of the powerful effect of a single dose of RMP, it was thought that a larger single dose of RMP might kill a significant amount of persisting viable organisms among skin-smear-negative MB patients, thereby reducing the relapse rate after stopping chemotherapy. To test the hypothesis, between 1982 and 1985 a single dose of RMP (1500 mg) was given to 136 MB patients before stopping chemotherapy. Before administration of RMP, all of these patients had become clinically inactive and skin-smear negative after dapsone monotherapy. Because many patients live near our institute, they often come for medical care. This provided an opportunity to follow up the patients for relapse. After nine relapses were detected after stopping chemotherapy, we thought it was important to define the magnitude of relapse among this particular group of patients. Therefore in 1992 we decided to examine all of the patients who could be retrieved.
The objective of this communication is to present the relapse rate, and to discuss the possible contribution of a single dose of RMP in preventing relapse among skinsmear-negative MB patients after dapsone monotherapy.
PATIENTS AND METHODS
Patients and treatment. Among the 136 patients, 91 were males and 45 females; in 1992 their mean age was 56 (range 24 to 78) years. They were diagnosed as MB leprosy because during the course of the disease they had had skin lesions of lepromatous or borderline leprosy, either by the Madrid classification or by the Ridley-Jopling classification, confirmed by histopathological examination of the skin biopsies; bacterial index (BI, Ridley scale) > 2+ at least in one site of the skin smears and a negative Mitsuda reaction. They had been treated with dapsone monotherapy, either by injection of an oil suspension twice weekly before 1970 or by oral administration of 100 mg daily afterward, for a mean duration of 14 (range 5 to 32) years. Between 1982 and 1985 when they were given RMP, they had already become clinically inactive (without active skin and nerve lesions) and skinsmear negative (at least one negative result). The 1500 mg single dose of RMP was given immediately before stopping chemotherapy and was administered under supervision by medical personnel.
Examinations during follow up. After stopping chemotherapy, when patients came to the clinic they received a thorough physical examination, especially the examination for leprosy. Skin smears were taken from at least six sites (including the same sites from which smears were taken in the past, and from suspected relapsed lesions, if any) if no such examination had been done within the last 12 months. During the screening for relapse in 1992, both examinations were performed on every retrieved patient regardless of whether he/she had been examined within 12 months.
Criteria for relapse. Relapse was suspected when an active skin lesion, i.e., diffuse infiltration, erythema, macula, plaque, nodule or leproma, was found and/or a BI > 2+ was detected from any site. Further examinations included repeating the physical examination, retesting the skin smears, taking a biopsy from the suspected skin lesion for histopathological examination, and testing the viability of the organisms by mouse foot pad inoculation (13). RMP susceptibility of the bacilli (13) was also determined in some patients. A relapse was confirmed if two of the following three criteria were met: a) BI > 2+ from any single site of skin smears, with or without an active skin lesion; b) histopathological examination showing active MB leprosy (LL or BL) structure, together with BI > 2 + ; and c) demonstration of viable organisms by mouse inoculation (13).
Statistical analysis. Data collection ended in June 1992. Using the number of relapsed cases after stopping chemotherapy as the numerator, the number of patients involved in the study and the duration of follow up, in terms of patient-years, were used as denominators for calculating, respectively, the overall relapse rate and the relapse rate per 100 patient-years. The duration of follow up for each individual patient was defined as the period of time between stopping chemotherapy and being discharged from the study, i.e., relapse, death, end of the study, or the last visit for the defaulters. Because patients entered the study between 1982 and 1985, by June 1992 the maximum duration of follow up ranged from 7 to 10 years. The "incubation period" of relapse was calculated as the number of months between stopping chemotherapy and the appearance of the relapse; the latter was defined as the midpoint between the dates of the last examination without relapse and the first examination revealing relapse (l3). In addition, the cumulative risk of relapse was analyzed using a life table (2) with 1-year intervals. Results were analyzed by the χ2 test, Fisher's exact probability calculation, and Student's t test. Differences were considered significant at the 95% level of confidence.
RESULTS
Out of the 136 patients in the analysis, 117 (86%) had been examined at least once after stopping chemotherapy, 72 (52.9%) had been examined at least once before screening and 84 (61.8%) were examined during screening, which included 45 cases who had never been examined before. Nineteen (14%) cases had never been seen after stopping chemotherapy. Ninety-two (67.6%) patients had been followed up for more than 5 years. The total duration of follow up was 708 patient-years, with a median duration of 83 months, or 6.9 years, per patient.
Altogether, 15 relapses were detected: 9 (60%) were detected during the follow-up period before 1992 and 6 (40%) were identified during screening. Neither the male/ female ratio nor the mean duration of dapsone monotherapy of these relapsed cases differed significantly from those of nonrelapsed cases. Active skin lesions were observed in all cases except one by the time relapse was diagnosed, and lepromas were detected in eight of them. As shown in Table 1, skin smear was, by definition, positive in all relapsed cases; the maximum BI (the highest BI among the sites of skin smears) was always > 4 + and the mean BI was 3.43. Solid-staining organisms were observed in 12 cases (80%), and the average morphological index (MI) of all relapsed cases was 5.1%. Histopathologic examinations of 13 of the 14 cases who were biopsied showed active LL or BL structures. Viable M. leprae were detected from 8 (72.7%) of the 11 cases from whom mouse foot pad inoculation results were available, and all three strains tested were susceptible to RMP. Slightly more than half of the relapses occurred later than 5 years after stopping chemotherapy, and the mean incubation period of all relapses was 56 ± 31 months, or 4.7 ± 2.6 years (Table 1).
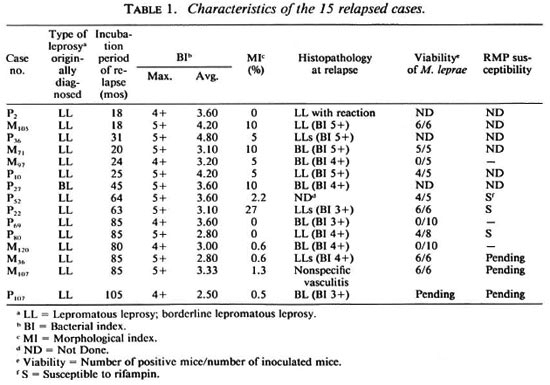
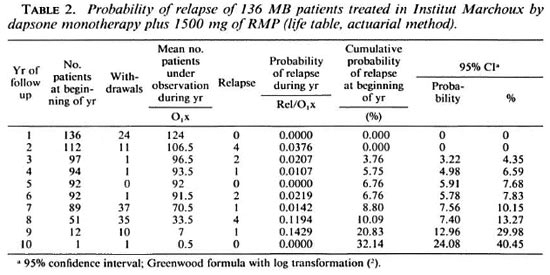
The overall relapse rate was 11%; the relapse rate per 100 patient-years was 2.1% with a 95% confidence interval of 1.1% to 3.2%. As shown in Table 2, the cumulative risk of relapse at year 7 of follow up was 8.8%, with a 95% confidence interval of 7.6% to 10.2%. Although the risk suddenly increased to 20.8% at year 9, its significance is doubtful because very few patients had been followed up that long.
DISCUSSION
It is understandable that the overall relapse rate is correlated with the duration of follow up. In the current study, because the duration of the follow up was only 7 to 10 years, the overall relapse rate (11%) was not very high as compared with other published (1, 3, 4, 10, 14, 15, 20). Nevertheless, the relapse rate per 100 patient-years in our study (2.1%) was among the highest(1,3,14). It may be more informative if one compares the cumulative risk of relapse after the same duration of follow up between different studies. The cumulative risk of relapse at year 7 of follow up was calculated to be 3.1% in Polynesia (4) and 6.4% Malaysia (20) as compared with 8.8% in the current study. Therefore, both the relapse rate per 100 patient-years and the cumulative risk of relapse among our patients were at least the same as in other patients who had received dapsone monotherapy only, suggesting that the administration of a single large dose of RMP could not prevent relapse or reduce its rate among patients who had already become clinically and bacteriologically negative after dapsone monotherapy.
In our earlier study, a group of 37 newly diagnosed MB patients were treated with a single large dose of RMP (1500 mg) plus daily dapsone (100 mg) for 24 months (13,19). The results of follow up indicated that in that particular group, the overall relapse rate was 20%, and the relapse rate per 100 patient-years was 3.6%(3). Because RMP was given at the beginning of chemotherapy, every patient received a fixed and relatively short (24 months) duration of dapsone monotherapy, and every patient was still skin-smear positive by the time chemotherapy was stopped. It is inappropriate to compare the results of relapse in that study with the current study. However, the high relapse rate again suggested that the administration of a single large dose of RMP at the beginning of dapsone monotherapy probably did not help much in preventing relapse.
The fact that a single large dose of RMP failed to prevent relapses in MB, especially lepromatous, patients by no means contradicted the observations that a single dose of RMP displayed significant bactericidal activity against M. leprae in mice (6-9) and in humans (12). The key issue is that the population size of viable organisms in a skinsmear-negative lepromatous patient is still far greater than the number of organisms which can be killed by a single dose of RMP. In a lepromatous patient, the number of viable M. leprae can be as much as 1010 or 1011 at the beginning of treatment, and may still be 105 when the skin smears become negative (11). Although a single dose of RMP killed about 90%-99% of the viable organisms (6-9), a significant number of viable organisms survived the killing and, therefore, caused a significant number of relapses if chemotherapy was stopped prematurely.
Acknowledgment. We thank Professors S. Pattyn (Prince Leopold Institute of Tropical Medicine, Antwerp, Belgium) and G. Discamps (Laboratoire d'Analyses de Biologie Médicale, Bordeaux, France) for histopathological examinations, and Professors J.-H. Grosset and B. Ji (Faculté de Medicine Pitie-Salpetricre, Paris, France) for their comments and assistance in editing.
REFERENCES
1. ALMEIDA, J. G., JESUDASAN, K., CHRISTIAN, M. and CHACKO, C. J. G. Relapse rates in lepromatous leprosy according to treatment regularity. Int. J. Lepr. 54(1986)16-20.
2. ARMITAGE, P. and BERRY, G. Survival analysis. In: Statistical Methods in Medical Research. 2nd edn. Oxford: Blackwell Scientific Publications, 1987, pp. 421-440.
3. BECX-BLEUMINK, M. Relapses in leprosy patients after release from dapsone monotherapy; experience in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Int. J. Lepr. 60(1992)161-172.
4. CARTEL, J.-L., BOUTIN, J. P., SPIEGEL, A., PLI-CHART, R. and Roux, J. F. Longitudinal study on relapses of leprosy in Polynesian multibacillary patients on dapsone monotherapy between 1946 and 1970. Lepr. Rev. 62(1991)186-192.
5. COLLABORATIVE EFFORT OF THE U.S. LEPROSY PANEL (U.S.-JAPAN COOPERATIVE MEDICAL SCIENCE PROGRAM) AND THE LEONARD WOOD MEMORIAL. Rifampin therapy of lepromatous leprosy. Am. J. Trop. Med. Hyg. 24(1975)475-484.
6. GROSSET, J.-H. and GUELPA-LAURAS, C.-C. Activity of rifampin in infection of normal mice with Mycobacterium leprae. Int. J. Lepr. 55(1987)847-851.
7. Ji, B., CHEN, J., Lu, X., WANG, S., NI, G., Hou, Y., ZHOU, D. and TANG, Q. Antimycobacterial activities of two newer ansamycins, R-76-! and DL 473. Int. J. Lepr. 54(1986)563-577.
8. Ji, B., PERANI, E. G. and GROSSET, J.-H. Effectiveness of clarithromycin and minocycline alone or in combination against Mycobacterium leprae infection in mice. Antimicrob. Agents Chemother. 35(1991)579-581.
9. JI, B., PERANI, E. G., PETINON, C. and GROSSET, J.-H. Bactericidal activities of single or multiple doses of various combinations of new antileprosy drugs and/or rifampin against M. leprae in mice. Int. J. Lepr. 60(1992)556-561.
10. KURTZ, X. M., DECLERCQ, E. E. and VELLUT, C. M. Rate and time distribution of relapses in multibacillary leprosy. Int. J. Lepr. 57(1989)599-606.
11. LEVY, L. Treatment failure in leprosy. Int. J. Lepr. 44(1976)177-182.
12. LEVY, L., SHEPARD, C. C. and FASAL, P. The bactericidal effect of rifampin on M. leprae in man: (a) single doses of 600, 900 and 1200 mg; and (b) daily doses of 300 mg. Int. J. Lepr. 44(1976)183-187.
13. MARCHOUX CHEMOTHERAPY STUDY GROUP. Relapse rates in multibacillary leprosy patients after stopping treatment with rifampin-containing combined regimens. Int. J. Lepr. 60(1992)525-535.
14. NEELAN, P. N. Relapse in lepromatous leprosy under sulphone treatment. Lepr. India 42(1972)98-102.
15. QUAGLIATO, R., BECHELLI, L. M. and MARQUES, R. M. Bacterial negativity and reactivation (relapse) of lepromatous outpatients under sulfone treatment. Int. J. Lepr. 38(1970)250-263.
16. QUINTO, R. S., CELLONA, R. V., FAJARDO, T. T. and DE LA CRUZ, E. C. Primary dapsone-resistant leprosy in Cebu, Philippines. Int. J. Lepr. 41(1981)427-430.
17. SHEPARD, C. C, LEVY, L. and FASAL, P. Rapid bactericidal effect of rifampin on Mycobacterium leprae. Am. J. Trop. Med. Hyg. 21(1972)446-449.
18. SHEPARD, C. C, LEVY, L. and FASAL, P. Further experience with the rapid bactericidal effect of rifampin on Mycobacterium leprae. Am. J. Trop. Med. Hyg. 23(1974)1120-1124.
19. SUBCOMMITTEE ON CLINICAL TRIALS OF THE CHEMOTHERAPY (THELEP) SCIENTIFIC WORKING GROUP O F THE UNDP/WORLD BANK/WHO SPECIAL PROGRAMME FOR RESEARCH AND TRAINING IN TROPICAL DISEASES. THELEP controlled clinical trials in lepromatous leprosy. Lepr. Rev. 54(1983)167-176.
20. WATERS, M. F. R., REES, R. J. W., LAING, A. B. G., FAN, K. K., MEADE, T. W., PAKISTAN, N. and NORTH, W. R. S. The rate of relapse in lepromatous leprosy following completion of twenty years of supervised sulfone therapy. Lepr. Rev. 57(1986)101-109.
1. M.D., Institut Marchoux, B. P. 251 Bamako, Mali.
2. D.Sc; Institut Marchoux, B. P. 251 Bamako, Mali.
Received for publication on 18 February 1993.
Accepted for publication in revised form on 2 March 1994.