- Volume 62 , Number 2
- Page: 215–9
Rate of relapse in multibacillary patients after cessation of long-course dapsone monotherapy supplemented by a final supervised single dose of 1500 mg of rifampin
ABSTRACT
When multidrug therapy was implemented in Senegal, 406 multibacillary (MB) patients who had been treated for more than 10 years by dapsone alone, and who had become clinically inactive and skin-smear negative, were released f rom treatment. Of these 406 patients, 298 were given a super vised single dose of 1500 mg of rifampin. Subsequently, 302 of them (229 who had been given rifampin and 73 who had not) were followed up by means of annual clinical and bacteriological examinations. Of the former 229 followed up for a mean period of 4.9 years, 34 patients relapsed (22 males and 12 females), giving a crude relapse rate of 15% and an overall risk of relapse of 3.1 per 100 patient-years. Of the latter 73 followed up for a mean period of 2.4 years, 5 relapsed (4 males and 1 female), giving a crude relapse rate of 6.8% and an overall risk of relapse of 2.9 per 100 patient-years. Such results, which are in agreement with those of a similar study conducted recently in Mali, indicate that the intake of a single dose of 1500 mg of rifampin by MB patients when they are released f rom long-course dapsone monotherapy does not result in a decrease of the relapse rate. Therefore, MB patients who have been treated with dapsone alone, even for long periods, should be put under multidrug therapy prior to their release f rom control.RÉSUMÉ
Lorsque la polychimiothérapie a été appliquée au Sénégal, 406 patients multibacillaires (MB), qui avaient été traités pendant plus de dix ans par la dapsone seule, étaient devenus clinquement inactifs et avaient des frottis cutanés négatifs, ont été libérés du traitement. Parmi ces 408 patients, 298 ont reçu une dose unique supervisée de 1 500 mg de rifampicin. Par la suite, 302 d'entre eux (229 qui avaient reçu de la rifampicine et 73 qui n'en avaient pas reçu) ont été suivis par des examens clinques et bactériologiques annuels. Parmi les 229 premiers, suivis pour une période moyenne de 4.9 ans, 34 patients ont rechuté (22 hommes et 12 femmes), ce qui donne un taux brut de rechute de 15% et un risque global de rechute de 3.1 pour 100 patientsannées. Parmi les 73 autres, suivis pour une période moyenne de 2.4 ans, 5 ont rechuté, donnant un taux brut de rechute de 6.8% et un risque global de rechute de 2.9 pour 100 patients-années. De tels résultats, qui sont en accord avec ceux d'une étude semblable réalisée récemment au Mali, indiquent que la prise d'une dose unique de 1500 mg de rifampicine par des patients MB lors de l'arrêt d'une monotháerapie de longue durée à la dapsone ne résulte pas en une diminution du taux de rechute. En conséquence, les patients MB traités par dapsone seule, même pour de longues périodes, devraient être mis sous polychimiothérapie avant d'être libérés des contrôles.RESUMEN
Cuando se implantó la poliquimioterapia (PQT) en Senegal, 406 pacientes multibacilares(MB) que habían sido tratados por más de 10 años sólo con dapsona y que habían llegado a ser clínicamente inactivos y baciloscópicamente negativos, fueron liberados del tratamiento. De estos 406 pacientes, 298 recibieron una dosis única de 1500 mg de rifampina (RMP). Subsecuentemente, 302 de los 406 pacientes (229 que habían sido tratados con RMP y 73 que no habían sido tratados con la droga) fueron vigilados y valorados anualmente por examenes clínicos y bacteriológicos. De los primeros 229 pacientes, sequidos durante un periodo promedio de 4.9 años, 34 mostraron recaídas (22 hombres y 12 mujeres), significando ésto una frecuencia de recaída del 15%, y un riesgo global de 3.1 por 100 pacientes/años. De los últimos 73 pacientes, seguidos druante un periodo promedio de 2.4 años, cinco tuvieron recaídas (4 hombres y una mujer), dando una frecuencia de recaída de 6.8%, y un riesgo global de recaída de 2.9 por 100 pacientes/años. Estos resultados, que están de acuerdo con los resultados de un estudio similar realizado en Mali, indican que la administración de una sola dosis de 1500 mg de RMP a los pacientes MB liberados de la monoterapia prolongada con dapsona no disminuye la frecuencia de recaída. Los pacientes MB que han sido tratados sólo con dapsona durante periodos prolongados de tiempo, deben someterse al tratamiento con poliquimioterapia antes de ser liberados del programa de control de la enfermedad.Following the recommendations of a World Health Organization Study Group (8), multidrug therapy (MDT) has been progressively implemented in Senegal since 1986. All leprosy patients suffering active disease and also those inactive but diagnosed before 1986 who had been treated with dapsone alone, for less than 10 years if multibacillary and less than 5 years if paucibacillary, have been put on MDT. All multibacillary (MB) patients who had received dapsone alone for more than 10 years and who had become clinically inactive and skin-smear negative were released from treatment. At that moment, a majority of them was given a final supervised dose of 1500 mg of rifampin. In 1993 a retrospective study was conducted in order to determine the rate of relapse observed in these patients during the years (1986-1993) following the cessation of treatment. The aims of this paper are to report the results of that study and to evaluate the impact, if any, of a final single dose of rifampin on the risk of relapse.
MATERIALS AND METHODS
Senegal, the westernmost country in western Africa, contained 201,000 sq. km with a population of 7 million inhabitants in 1993. It is divided into 10 administrative and medical regions; each region is divided into three medical districts. In the main town of each region 2-3 specialized nurses and a laboratory technician are in charge of the leprosy control program under the supervision of a medical doctor responsible for the public health activities in the region. The medical coordinator of the program is based at the Direction of Public Health Services in Dakar, the capital of Senegal. The Institut de Léprologie Appliquée is also located in Dakar. With respect to public health activities, the institute is responsible for the supervision of the laboratory examinations performed in the 10 regions and for the training of the physicians, the specialized nurses, and the laboratory technicians involved in the program.
Since the implementation of the control program in Senegal by the end of the 1970s, the diagnosis of leprosy has been based on a clinical examination of the skin and large nerve trunks and a search for acid-fast bacilli (AFB) in earlobes and skin lesions. Dapsone was the basis of treatment and was prescribed as a life-long monotherapy for MB patients and for an average of 10 years for paucibacillary (PB) patients.
Between January 1986 and December 1989, in 5 of the 10 regions MB patients who had been treated with dapsone alone for more than 10 years were examined, and skin smears were taken from the earlobes and skin to search for AFB. All those who were found clinically inactive and smearnegative were released from treatment and part of them were given a supervised single dose of 1500 mg of rifampin. Subsequently, they were examined every year, and smears were taken systematically from all of them at each annual examination. A similar assessment was performed for those who presented between two annual examinations with clinical evidence of relapse. For the purpose of the study, a relapse was defined as the reappearance of AFB with a bacterial index (6) > 2 in at least one sample, accompanied by clinical signs.
Each year of the study patients were withdrawn in the case of a) relapse, b) reinstitution of treatment, c) death or disappearance, or d) exclusion from observation (because all patients were not released from treatment the same year, the duration of follow up was not the same in each of the five regions). Each withdrawn patient was considered to have been excluded at the end of the year in which the event occurred. Each patient entered into the study (starting date or year 0) when dapsone monotherapy was stopped, with or without final simultaneous intake of rifampin. The closing date of the study was December 1993.
RESULTS
Of 568 MB patients who had been treated with dapsone alone for more than 10 years, 162 were found to have clinical and bacteriological evidence of active disease and were put on MDT. The remaining 406 patients were released from monotherapy, 298 of whom were given a final supervised dose of 1500 mg of rifampin. By December 1993 data were available for 302 of the 406 patients: 229 (141 males and 88 females) who had been given rifampin and 73 (43 males and 30 females) who had not been given any supplementary treatment. The first group of 229 patients was followed up for a period ranging from 3 to 8 years (mean 4.9 years); the second group of 73, for a period ranging from 2 to 5 years (mean 2.4 years). Sixteen patients in the first group and 30 in the second group were withdrawn from the study because they were lost to control or because they died before December 1993.
Among the 229 patients who had been given a single dose of rifampin, 34 suffered a relapse, the diagnosis of which was made by means of the annual examination in 23 cases (68%). The earliest relapses were observed during the first year of follow up and the final one during the sixth year. A total of 34 relapsing patients (22 males and 12 females) gives a crude relapse rate of 15%; the relapse rate was 13.6% among the female patients and 15.6% among the male patients. As shown in Table 1, the cumulative number of patient-years of follow up was 1113, and the overall risk of relapse was 3.1 per 100 patient-years. The annual risk of relapse ranged from 0.9 to 6 per 100 patient-years between year 1 and 6 after release from treatment, and no significant difference was found between the six successive annual risks of relapse (p > 0.05).
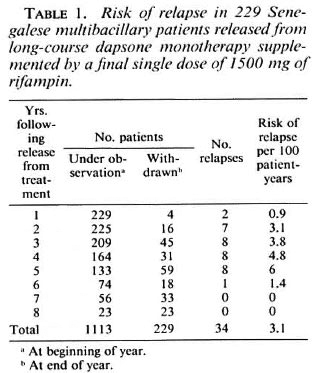
Among the 73 patients released from dapsone monotherapy without any supplementary treatment, 5 (4 males and 1 female) suffered a relapse (2 during each of the first 2 years of follow up and 1 during the third year), giving a crude relapse rate of 6.8%. The relapse rate was 3.3% among the female patients and 9.3% among the male patients. As shown in Table 2, the cumulative number of patient-years of follow up was 170, and the overall risk of relapse was 2.9 per 100 patient-years, not significantly different (p > 0.05) from that of 3.1 observed in the first group. The annual risk of relapse ranged from 2.6 to 3.8 per 100 patient-years during the first 3 years of follow up, and no significant difference was found between the three successive risks of relapse (p > 0.05).
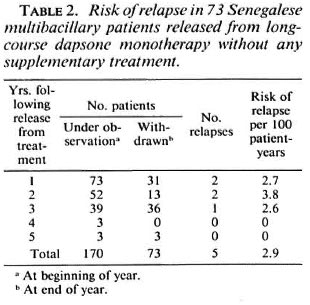
Finally, if considering only the first 3 years of follow up in each group, the overall risk of relapse was 2.6 per 100 patient-years in the first group during that period, not significantly different (p > 0.05) from that of 3 per 100 patient-years observed in the second group during the same period.
DISCUSSION
Our data were collected from routine activities and, as in any retrospective study, caution has to be taken in the interpretation of the findings. Also, it is to be noted that the number of included patients and the duration of follow up were much smaller in the group released without any supplementary treatment (control group) than in the group given rifampin. Nevertheless, it appears clear that the risk of relapse was not smaller in the latter than in the former, at least during the first 3 years of follow up. Moreover, the crude relapse rate of 15% and the overall risk of relapse of 3.1 per 100 patient-years observed after a mean follow up of 5 years in the group given rifampin are comparable to, or slightly higher than, those reported either by Waters (7) in Malaysia (8.6% and 1.04 per 100 patient-years) or by Becx-Bleumink (1) in Ethiopia (13.2% and 2.48 per 100 patient-years) after follow up of similar duration (9 and 7 years, respectively) of MB patients released from long-course dapsone monotherapy (20 years and more than 10 years, respectively). Actually, despite the small size of our control group and despite the fact that results of different studies performed in different countries are difficult to compare, a most important finding is that in our MB patients who received a final supervised dose of 1500 mg of rifampin, the overall risk of relapse was not lower than that observed in MB patients released from long-course dapsone monotherapy (in Senegal or in other countries) without any supplementary treatment.
In Mali also a supervised single dose of 1500 mg of rifampin was given to MB patients who had become clinically inactive and skin-smear negative after long-course dapsone monotherapy and who were then released from treatment. In 136 patients followed up for 7 years the crude relapse rate was 11% and the risk of relapse was 2.12 per 100 patient-years (4). From those results, similar to ours, the authors of the study have concluded that the administration of a single dose of 1500 mg of rifampin was not able to prevent the occurrence of relapse. The results of the present study and those of the study conducted in Mali are in agreement, indicating that the intake of a dose of 1500 mg of rifampin by MB patients when they are released from long-course dapsone monotherapy does not result in a decrease of the relapse rate.
Finally, from our data the question arises whether MB patients who have been treated with dapsone alone, even for long periods (10-15 years or more), could be released without any supplementary treatment. The results of studies performed in several countries have indicated that relapsing MB patients can constitute a high percentage of active cases of leprosy in a population and can represent an important source of infection (3-5). Moreover, it is known that an important proportion of MB patients relapsing after a long course of dapsone monotherapy harbor Mycobacterium leprae strains fully resistant (more than 50% in Caribbean patients), or having some degree of resistance, to dapsone (3,7). Therefore, as ecommended by several author (1,2,5,7) and as done in some leprosy control programs (5), MB patients who have been treated with dapsone alone should be put on MDT prior to their release from control.
REFERENCES
1. BECX-BLEUMINK, M. Relapses in leprosy patients after release from dapsone monotherapy; experience in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Int. J. Lepr. 60(1992)161-172.
2. CARTEL, J.-L., BOUTIN, J.-P., SPIEGEL, A., PLICHART, R. and Roux, J.-F. Longitudinal study on relapses of leprosy in Polynesian multibacillary patients on dapsone monotherapy between 1946 and 1970. Lepr. Rev. 62(1991)186-192.
3. CARTEL, J.-L., NAUDILLON, Y. , REMY, J.-C. and GROSSET, J.-H. Contribution of relapses to total infection sources of leprosy in Guadeloupe. Lepr. Rev. 58(1987)339-348.
4. JAMET, P., BLANC, L., FAYE, O. , TRAORE, I. and BOBIN, P. Single dose rifampin cannot prevent relapse in skin-smear negative multibacillary leprosy patients after dapsone monotherapy. (Abstract) Int. J. Lepr. 61 Suppl. (1993) 6A.
5. LI, H.-Y . Problems of leprosy relapse in China. Int. J. Lepr. 61(1993)1-7.
6. RIDLEY, D. S. Bacterial indices. In: Leprosy in Theory and Practice. Cochrane, R. G. and Davey, T. F., eds. Baltimore: Williams and Wilkins, 1964, pp. 620-622.
7. WATERS, M. F. R., REES, R. J. W., LAING, A. B. GKHOO, K. F., MEADE, T. W., PARIKSHAM, N. and NORTH, W. R. S. The rate of relapse in Lepromatous leprosy following completion of twenty years of supervised sulfone therapy. Lepr. Rev. 57(1986)101-109.
8. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. M.D., Director.
2. M.D., DAHW/Institut Léprologie Appliquée, B. P. 11023, Dakar CD, Senegal.
Received for publication on 7 December 1993.
Accepted for publication in revised form on 24 February 1994.