- Volume 62 , Number 2
- Page: 299–301
Assessment of the purity of M. leprae preparations f rom tissues of leprosy-infected laboratory animals
To the Editor:
Mycobacterium leprae isolated from the tissues of experimentally infected animals must be checked for tissue contamination if they are destined for diagnostic or vaccine preparations. Available methods for assessment of M. leprae purity at different steps of their purification, such as light and electron microscopy (4), immunoblotting and ELISA (for detection of armadillo liver protein) (7), electrophoresis of M. leprae sonicates (determination of electrophoretic mobility of host malate dehydrogenase) (9), or skin tests on guinea pigs presensitized with tissues from intact animals (3), are cither of low sensitivity (9) or are complicated, multistep and time-consuming (3,8,9).
We propose to assess the purity of M. leprae isolated from the tissues of experimentally infected animals by using a method of gas chromatography of copyrolysates of mycolic acids forming a part of mycobacterial cell walls (1). Esterified residues of the side chain of destructed mycolic acids extended by two carbon atoms eluate from the column. Previously we have shown (6,7) that mycolic acid residues with 22, 24, 26, and 28 atoms of carbon were detected on copyrolytic chromatograms of M. leprae only; whereas on chromatograms of normal animal tissues mycolic acids and other longchain components (with over 20 carbon atoms) were absent. Thus, the presence of short-chain components on the copyrolytic chromatograms of M. leprae undergoing purification may suggest host tissue contamination of the mycobacterial preparation. On copyrolytic chromatograms of M. leprae passed on mice and rats, tetracosanic acid (C24:0) prevails; whereas in M. leprae isolated from human lepromas and in M. leprae once passed on armadillos, behenic acid (C22:0) predominates among mycolic acids (The Figure).
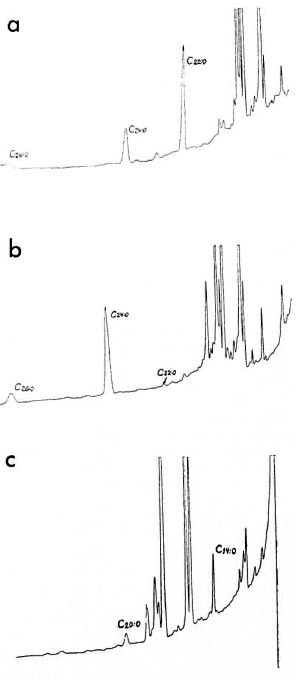
The figure. Chromatograms of methyl ethers of fatty acids of M. leprae and foot pad tissue from mice: a) M. leprae once passed on armadillo and purified from infected spleen, peak of behenic acid (C22:0) prevails; b) M. leprae (3rd passage) isolated from mouse foot pads, peak of tetracosanic acid (C24:0) prevails; c) foot pads of intact mice, peak of tetradecanoic acid (CI 4:0).
For a quantitative assessment of the extent of tissue contamination of M. leprae preparations, we propose to determine the ratio between the amounts of the mycolic acid prevailing in a given mycobacterial strain and tetradecanoic acid (CI4:0), the content of which is approximately equal in animal tissue and in mycobacteria (l%-2% of total acids) and the ratio of tetradecanoic acid in mycobacteria to that in animal tissues therefore may be assumed as 1.
Infected foot pads of mice and rats and spleens and lepromas from experimentally infected armadillos were used. M. leprae were purified from the tissues according to the methods proposed by Prabhakaran (5) and Draper (2). Draper's purification of a part of the preparations was ended at the stage of separation in a gradient of Percoll (variant 1), while the remaining preparations were put through all the steps of purification including the two-phase polymeric system (variant 2).
The copyrolytic method (1) allows one to obtain methyl ethers of fatty acids without previous lipid extraction of the bacterial mass, thus cutting analysis time to 1-2 hr.
Purified M. leprae (5-10 mg) were placed in a glass ampoule into which 0.1-0.2 ml of a 7% solution of tetramethyl ammonium hydroxide in 80% aqueous methanol was added. The ampoule was soldered and heated at 120ºC-130ºC for 20-30 min. The sample took on the appearance of a transparent or slightly opalescent solution. Then 2-3 ml of the solution was introduced into the evaporator of a gas chromatograph with a flame-ionization detector and steel columns (1.5-2 m x 3 mm internal diameter), packed with celite 545 (60-80 mesh) and with 7% apieson L as a liquid phase. Analytical conditions were as follows: the temperature of the column thermostat was programmed from 170ºC to 270ºC at a rate of 5ºC/min; the temperature of the injector was held at 360ºC; argon was used as a gas carrier; argon and hydrogen flow was 2.4 1/hr, air flow was 25 1/hr.
The Table shows the results of gas chromatographic assessment of host tissue contamination of M. leprae as judged by the ratio of the prevailing mycolic acid to tetradecanoic acid. The degree of tissue contamination varied from 4.3 to 15.2, depending on the method of purification. The highest values corresponded to M. leprae preparations purified by Draper's method (variant 2), involving separation in a two-phase polymeric system. The low indices were attributed to M. leprae preparations purified by Prabhakaran's method.
It is important that a choice of optimal ratios of the prevailing types of mycolic acids (in our case, C24:0 and C22:0) to tetradecanoic acid (CI4:0) depends on the tasks of the experiments and the demands for purity of the preparation.
- Margarita N. Dyachina, M.D.
Senior Scientific Worker
Leprosy Research Institute, GSP-7
Astrakhan 414000, Russia
- Ludmila M. Pynchuck, Dr. Biology
Senior Scientific Worker
- Alla L. Lazovskaya, M.D.
Professor and Head
Animal Tuberculosis Laboratory
Research Institute of Veterinary Science
Nyshnyi Novgorod, Russia
- Anatoly A. Juscenko, M.D.
Director
Leprosy Research Institute, GSP-7
Astrakhan 414000, Russia
REFERENCES
1. ANDREYEV, L. V. and SKLIFAS, A. N. Application of tetramethyl ammonium hydroxide for determination of total fatty acid composition in microorganisms. Trans. Acad. Sci. U.S.S.R. Biol. Ser. 1(1979)95-102.
2. DRAPER, P. Problem related to purification of M. leprae from armadillos tissues and standardization of M. leprae preparation. Report on the Enlarged SC Meeting, Geneva, 1979. WHO Document, annex 1, protocol 1/79, p. 4-4.
3. DYACHINA, M. N., P ERVUKHIN, Y. V., V OROBYEVA, Z. G., ET AL. [Separation of M. leprae and M. lepraemurium from the tissues of leprosy-infected animals.] Materials of Karakalpak Republic Scientific Conference on Leprosy, Nukus, 1977, pp. 24-26.
4. GAN, S.-C. Purification of M. leprae isolated from human skin biopsies. (Letter) Int. J. Lepr. 54(1986)475-476.
5. PRABHAKARAN, K., HARRIS, E. B. and K IRCHHEI-MER, W. F. Metabolic inhibitors of host tissue origin in Mycobacterium leprae. Lepr. India 51(1979)348-357.
6. PYNCHUCK, L. M., DYACHINA, M. N. and LAZOVSKAYA, A. L. [Gas-liquid chromatography of fatty acids of .M.leprae passed on animals.] Aktualnye Problemi Leprologii Astrakhan (1978)97-100.
7. PYNCHUCK, L. M., D YACHINA, M. N. and L AZOVSKAYA, A. L. [High fatty acids of M. leprae, isolated from animal tissues with experimental leprosy.] Aktualnyc Problemi Leprologii Astrakhan (1984) 8-12.
8. QUESADA-PASCUAL, F., GILLIS, T. P. and BUCHANAN, T. M. Quantitation and characterization of armadillo protein that contaminates Mycobacterium leprae during its purification. Int. J. Lepr. 49(1981)506-510.
9. REZAEV, A. A., RYZHOVA, N. Y. and DYACHINA, M. N. A method for estimating tissue contamination of M. leprae isolated from infected animal tissues. Inventor's certificate N 1482398 (1989).
Reprint requests to Dr. Juscenko.