- Volume 62 , Number 2
- Page: 301–4
"Tuberculoid contamination" in histoid hanseniasis
To the Editor:
In their letter reporting a patient with histoid leprosy, Fiallo, et al. (3) mention that the phenomenon of "tuberculoid contamination" in this condition, although not satisfactorily explained, would suggest a hyperactive cellular response.
If a focal hyperactive response to true Hansen's bacilli (Mycobacterium leprae) is meant, it is difficult to understand why this type of pathology should appear in the same individual whose tissues otherwise show such complete anergy to the organism that is found in excessive numbers-more even than in most patients with ordinary active polar Virchowian hanseniasis (lepromatous leprosy).
In a detailed paper on histoid leprosy, Rodriguez (7), while not specifically referring to "tuberculoid contamination," does mention that in his Case 10 a histological examination showed spindle-shaped histiocytes occurring in small discrete nodules, in one of which small foci of epithelioid and giant cells were seen. He speculates that the Hansen's bacilli (some of which appeared to be longer than normal) might have been mutants. However, Jopling (4) points out that although some authors have stressed that the bacilli in the spindle-shaped histiocytes may be longer than normal, others have found little to differentiate them histologically and bacteriologically from hyperactive lepromatous nodules [our italics]. The question "Active or hyperactive against exactly what?" generally remains unasked.
It is clear from Wade's original description (10) that, apart from their histology, his foci of "tuberculoid contamination" are histopathological entities having nothing else in common with polar tuberculoid macules in which true Hansen's bacilli are known to have a-possibly indirect-causative role and which are large enough to be clinically identifiable.
We believe that mutation of Hansen's bacilli is involved, but in the formation of the "contamination" foci themselves rather than in the production of the mycobacteria seen in the histoid nodules as suggested by Rodriguez (7).
One of us [Corcos, M. G. Molecular biology of H.D., the case for the involvement of a transferrable plasmid. (9 parts) The Star 40 (1981) 6-41 (1982) 16] proposed that gene rearrangements involving changes between integration, autonomy and extrabacillary transfer of (foreign) plasmid genes are responsible for some otherwise unexplained findings in HD. These are: a) failure of Hansen's bacilli to replicate in vitro and almost certainly ex vivo; b) lack of histologically evident axoplasmic and skin damage and the presence of the "clear zone" in locations where and at times when Hansen's bacilli clearly are replicating; c) progressive and resolving skin and axoplasmic damage when and where Hansen's bacilli are not only failing to replicate but often are not even present in quantities detectable by any staining methods; d) the ultraccllular nature of "lepra reactions"; e) the bacteriolysis of Hansen's bacilli by smaller walled particles noted by Wade (9); f) the cell wall deficient L forms described by Chatterjec (2) and by some other investigators; g) the specific Mitsuda-negative response of polar Virchowian (lepromatous) human hosts, as well as their nonspecific, epithelioid and giant cell response to the presence of injected cultivable mycobacteria; and h) the "tuberculoid contamination" that is the subject of this letter.
It is perhaps worth mentioning that one of us [Corcos, M. G. Molecular biology of H.D., the case for the involvement of a transferrable plasmid. Part 8. The Star 41 (1982) 7] using (e) and (f) above, was able to predict on theoretical grounds the presence of a transposon in every Hansen's bacillus. Recently, Keer, et al. (5) described in its genomic DNA, an element unique to M. leprae with repeats arranged in an inverted array separated by 400 bp, suggesting that it may be a transposon.
We propose that the "tuberculoid contamination" of histoid hanseniasis (lepromatous leprosy) is due to the extremely rapid replication of genotypically and phenotypically true Hansen's bacilli causing occasional gene loss so that these prokaryotes become cultivable mycobacteria to which even polar Virchowian (lepromatous) skins produce nonspecific, nonprogressive foreign body responses. How this might come about is explained in the diagram -not drawn to scale -originally published in The Star [41 (1982) 7] reproduced here (The Figure) by kind permission of the Editor. Note how the (circular) plasmid on becoming autonomous now carries only the NI (Neuron Invasion) gene (star) proposed by Barksdale (1), leaving the shared replicator gene (square) and the protein coat and all other cell-wall coding genes (black circular disc) on the (elliptical) mycobacterial chromosome. The true Hansen's bacillus has been drawn without its "foreign" surface markers (F F F F F F) since they are not recognized by the Virchow cell (V) although they are, in fact, present at all times; (a) shows the situation immediately after the (Virchow cell induced) mycobacterial replication, (b) shows the situation we would expect to find if the Virchow cell has died (dotted lines) or if the bacilli have been extruded. With no functioning Virchowian macrophage recognition and effector system (black triangle) in place, the Hansen's bacillus has reverted to a state of dormancy, the chromosome being deprived of the replicator gene which, as always, remains part of the autonomous plasmid. However, the daughter cell, which is losing by dilution its nonreplicable NI gene-harboring plasmid, is now able to, and does, undergo fission outside the Virchow cell of a polar Virchowian (lepromatous) patient.
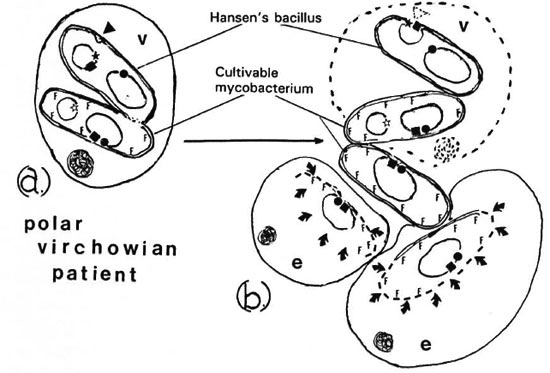
The figure. Hyopthetical M. leprae plasmids/ V = Virchow cell; e = epitheloid cell; 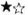 = neuron invasion (NI) gene of Barksdale; ● = protein coat and other cell-wall coding genes; ■ = (shared replicator gene; FFFF = foriegn surface markers; ◄ = recognition and effector systems of Virchow
= neuron invasion (NI) gene of Barksdale; ● = protein coat and other cell-wall coding genes; ■ = (shared replicator gene; FFFF = foriegn surface markers; ◄ = recognition and effector systems of Virchow 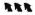 = digestion by epitheloid cells;
= digestion by epitheloid cells;  = eukaryote cell nucleus.
= eukaryote cell nucleus.
The patient's multipotential macrophages now become actual epithelioid cells (e) which recognize the unmasked "foreign" surface markers of these replicating cultivable non-Hansen mycobacteria and begin to digest them (arrows), engendering the minute foci of nonprogressive "tuberculoid contamination." The result of the operation of the recognition and effector mechanisms of the Virchow cell on each true Hansen's bacillus is the prevention of lateral transfer of the plasmid to axoplasm, where it would otherwise retain its virulent NI gene and replicate. This, we believe, is just what happens in Virchowian Hansen's disease (8) (secondary neural lepromatous leprosy) when the molecular defense, for various reasons, fails. Harmless replication of each true Hansen's bacillus, begetting two daughter cells, follows when the autonomous plasmid, as a result of a successful Virchow cell defense, integrates with the otherwise defective mycobacterial genome (integration not shown in this diagram). It continues as an epiphenominal self-perpetuating feedback loop when two replicated plasmids revert to autonomy in two daughter cells. It is noteworthy that long-term axoplasmic damage in (Mitsuda-negative) Virchowian patients may occur in the absence of any kind of epithelioid and giant cell histology, but contemporaneously with the disappearance of Hansen's bacilli: also, that after more than a century of observation, significant amounts of axoplasmic damage have not been found to be associated with cultivable mycobacteria.
The functional tuberculoid process manifested by hansenian damage which may occur at virtually the same place and time as Virchowian hanseniasis-giving rise to all the intermediate and borderline forms-is not explicable at the molecular level as "cell mediated immunity." It is more like a nonspecific, progressive, autodestructive and scavenging activity against already genetically infected axoplasmic nexus and basal skin cells, mounted by those human hosts able to do so. In contrast to the presence of the "clear zone" seen in polar Virchowian hanseniasis, its absence from regions of polar tuberculoid hansenian damage is striking.
At present, the idea that every "healthy" Hansen's bacillus seen under light or electron microscopy-including those accidentally transferred from one person to another-is the result of a successful human host initiated molecular defense process must be difficult for caring clinicians and field workers to accept. There are, however, increasing numbers of observations in support of it. The correct interpretation of these observations is, we believe, being retarded by the use of are haic terminology such as "leprosy" and words with the prefix "lepr. . . ." These are all-embracing historical and sociological terms no matter what they are intended to mean, and it is difficult to see how they can have relevance to a rational understanding of ontogeny and pathogenesis at ultracellular and macromolecular levels. This is why we prefer the (slightly modified) terminology of Rotberg (8) to the conventional one.
We have already drawn attention to the doubtful value of "cell-mediated immunity" which, it seems, is applicable to both tuberculoid and Virchowian processes according to the inclination of the author employing the term. Another disadvantageous designation is "auto-immunity," since it appears to be used to describe what is, in fact, autodestruction or autogenous damage to tissues for no presently discernable reason, and leaves unasked questions about molecular dynamics posing only those concerning structural biology. To us, neither "cell-mediated immunity" nor "autoimmunity" adequately describes the etiology and pathogenesis of polar tuberculoid Hansen's disease or of "tuberculoid contamination" of histoid hanseniasis.
Does an attempt at interpreting cellular, clinical and epidemiological observations in molecular terms really matter, especially when the forgetting of much orthodox dogma is involved? Technicians and industrialists clearly think that some forgetting is of importance in improving their results (6), and in this we agree with them. We would go further and suggest that unless the art of hermeneutics is rigorously and creatively applied to the extensive and ever-growing mass of discoveries and of negative and inconclusive experiments in HD, much biochemistry will be unexplained, many paradoxes will persist unresolved, clinical and histological findings will continue to be largely descriptive, and prophylaxis and therapy will remain empirical into the foreseeable future.
Whether or not current misunderstanding and misinterpretation of accumulating data will have any effect on the ultimate eradication of HD remains to be seen. We think they will have an adverse one.
- Michael G. Corcos,
M.R.C.S., L.R.C.P., D.T.M.&H.
6 Grange Avenue
Beeston
Nottingham NG9 1GJ
England
- Christopher D. Corcos,
M.R.C.Psych., B.Sc, M.B.B.S.
Hillcrest Hospital
P.O. Box 233
Greenacres
S.A. 5086
Australia
REFERENCES
1. BARKSDALE, L. Concerning the NI (neuron invasion) gene of Mycobacterium leprae. Int. J. Lepr. 47(1979)358-359.
2. CHATTERJEE, B. R. Growth habits of Mycobacterium leprae, their implications. Int. J. Lepr. 33(1965)551-555.
3. FIALLO, P., NUNZI, E., BETTO, P., BONALDI, E. and TORREGROSSA, F. Histoid leprosy in early macular lepromatous leprosy; incidental finding or sign of augmented local immunity? Int. J. Lepr. 61(1993)471-472.
4. JOPLING, W. H. Handbook of Leprosy. London: William Heinemann, 1978, p. 33.
5. KEER, J., DE S MET, K., GELLES, D. and STOKER, N. Characterization of a duplicated sequence in Mycobacterium leprae. Trans. R. Soc. Trop. Med. Hyg. 87(1993)344.
6. MADDOCK, I. Why industry' must learn to forget. New Scientist 93(1982)368-370.
7. RODRIGUEZ, J. N. The histoid leproma, its characteristics and significance. Int. J. Lepr. 37(1969)1-21.
8. ROTBERG, A. Notice to contributors. Hansenol. Int. 2(1977) inside front cover.
9. WADE, H. W. "L" bodies or protoplasts of the leprosy bacillus? Int. J. Lepr. 30(1962)501-503.
10. WADE, H. W. The histoid variety of lepromatous leprosy. Int. J. Lepr. 31(1963)129-142.