- Volume 62 , Number 2
- Page: 284–92
Prospects of global elimination of leprosy as a public health problem by the year 2000
The World Health Assembly, in May 1991, approved a resolution about the global elimination of leprosy as a public health problem by the year 2000, and defined this elimination as the reduction of prevalence to 1 case or less per 10,000 inhabitants. The resolution took into account the considerable progress achieved with multidrug therapy (MDT), with great reduction of prevalence as well as the substantial support of nongovernmental organizations and the fact that many countries were giving higher priority to leprosy control.
"The estimative number of leprosy cases world-wide in 1991 was 5.5 million. This figure shows a marked decline from the 10-12 million cases estimated earlier and suggests that it is the success of MDT that brought such a drastic reduction."1
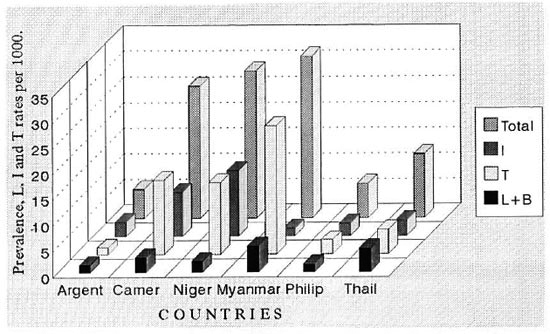
The figure. Prevalence, L, I and T rates per 1000 in Myanmar (BCG trial area) and in some other areasof the world (WHO random sample surveys).24 Argent = Argentina; Canter = Cameroon; Niger = Nigeria;Philip = The Philippines; Thail = Thailand.
Noordeen2 analyzed in detail the relevant findings and the justifications for the elimination strategy as well as the limitations of MDT.
The possibility of reaching the elimination goal depends mainly on three factors and related conditions.
1. Antileprosy drugs and MDT
It is known that the advent of sulfones3 represented a remarkable progress, with revolutionary changes in leprosy control: abolishment of leprosaria and shift from inpatient to outpatient care. Early diagnosis and early chemotherapy in dispensaries became the most important method of control, supported by health education and relevant measures. For more than 30- years International Congresses of Leprology and WHO Expert Committees on Leprosy considered sulfone the drug of choice in the treatment of leprosy. Gradually it was recognized that their main shortcoming was their slow effect (clinical, bactériologie and histologic) in the multibacillary forms of leprosy. Because of the long period of treatment required for lepromatous (L) and borderline (B) patients, a large proportion of them became irregular or lost sight of. To this must be added the high proportion of inactive cases who relapsed, due to the persistence of bacilli, and also the development of resistant Mycobacterium leprae strains, as confirmed by mouse foot pad inoculation.
The WHO/MDT scheme (dapsone + rifampin + clofazimine) is at present considered the best one: it increases the efficacy of treatment and reduces the risk of development of resistant strains of M. leprae.
Attention should be drawn to Dietrich's, et al.4 prospective randomized multicentric trial. They compared monotherapy (dapsone, DDS) and combined therapy: DDS + rifampin (RMP) and DDS + rifampin + isoniazid (INH) + prothionamide in 307 LL and BL patients. After 3 years, treatment was stopped and 216 patients finally could be evaluated. Several treatment parameters were adopted including relapses and patients with established DDS resistance in the mouse foot pad. All of them did not show any significant differences among the three treatment regimens. .. . It is concluded that DDS is very efficient, rifampin or the combination with rifampin, INH and prothionamide do not add substantially to the treatment success. . . . However the final conclusion can only be made after the termination of the 5-year treatment-free period.
In another trial by Irudayaraj and Aschhoff,5 132 untreated leprosy patients (77 LL + 55 BL) were randomized into three different regimens: 1) DDS; 2) DDS + RMP and 3) RMP + Isoprodian and treated continuously for 3 years. Pre- and post-treatment biopsies were inoculated into the mouse foot pad. "The BI reduction between the regimens was not significant. The percentages of bacteriologically negative patients after 3 years period in these regimens were not different from each other. Eighty patients (63%) remained still BI positive at the end of the study. About 96% of the strains from bacteriologically positive patients after 3 years' treatment did not multiply in the mouse foot pad, indicating so far a uniform kill after this period. No relapses have been encountered."
In a THELEP trial, in connection with UNDP and WHO6 combined drug regimens were studied in 215 previously untreated patients with lepromatous leprosy in Bamako, Mali, and in Chingleput, India. "The regimens -daily RMP, DDS and clofazimine (CLOF) or prothionamide (PTH); a single initial large dose of RMP together with daily DDS; and daily CLOF or PTH for the first 3 months, together with daily DDS and RMP, either in a single initial large dose or 900 mg once weekly-were administered for 2 years. During this time, biopsy specimens were obtained, and the recovered M. leprae were inoculated into thymectomized-irradiated (TR) mice for detection of persisters. In addition, periodically the bacterial index (BI) and logarithmic index of biopsies (LIB) were measured, the patients were examined clinically and observed for side effects, and a number of laboratory tests were carried out.
"Despite the widely varying 'strength' of the experimental regimens no differences were demonstrated among the regimens, with respect to the frequency with which persisting M. leprae were detected, clinical response, and adverse reactions, with two exceptions."
It should be noted that with the regimens of a single initial large dose of RMP together with daily DDS for 2 years the results were similar to those of other combinations of drugs, including one with daily RMP and CLOF! Side effects should also be taken into account.
Further results of these studies are expected with great interest, mainly regarding relapses, as well as the findings of other trials concerning MDT and new drugs. Ofloxacin, minocycline and clarithromycin are being tried in pilot studies with promising results, but have lower bactericidal activity than rifampin. It is hoped that other MDT schemes with the new drugs will have greater impact on the disease, shortening the period of treatment and reducing the rate of relapses.
Notwithstanding the findings of the three trials, the WHO/MDT as well as other combinations must be applied because of the risk of development of M. leprae -resistant strains with monotherapy. It should be recognized that, in spite of the progress achieved, at present we are far from having an ideal single drug or combined regimens for leprosy treatment. The efficacy of MDT may be improved by replacing CLOF with ofloxacin or another drug. CLOF is not accepted by a certain number of patients due to the pigmentation it causes.
So far the similarity of findings of various regimens of MDT and DDS alone does not suggest that the drastic decline of prevalence since 1982 is due to the success of MDT. Instead, two other causes should be considered. When MDT was adopted (1982) the duration or treatment was considerably shortened: 6 months for the paucibacillary (PB) cases, 2 years for the multibacillary (MB) cases. Previously the duration was 3 - 5 years for the former and 5-10 years or more for the latter. The anticipated release from control of all cases -PB and MB- automatically caused the decrease of prevalence. The Figure shows WHO data regarding mass survey (Myanmar) or random sample surveys in several countries (Argentina, Cameroon, Nigeria, The Philippines and Thailand) with total prevalence rate as well as lepromatous (L) + borderline (B), tuberculoid (T) and indeterminate (I). These are much higher for T and I cases than L cases. The considerable shortening of the treatment period and follow up caused a drastic reduction of total prevalence and for each form of leprosy. To this should be added that WHO/MDT was implemented with an improvement of the control program in each area. When this is accomplished, even with monotherapy, appreciable results have been achieved in several areas of the world. The prevalence was also greatly reduced because patients were released from control after long periods of treatment (usually 5- 10 years for LL cases).
The prospective of obtaining such a decrease of prevalence with monotherapy had been indicated by Martinez and Bechelli7 regarding tropical Africa, in the areas where the socioeconomic situation is favorable and there is an effective control program. Since there is, mainly in Africa, a very high proportion of tuberculoid cases -in whom spontaneous regression of lesions occurs in 60%-80% of cases,8,9 and relapses are rare- an effective campaign could stabilize and reduce the prevalence to a variable degree in a relatively short period.
Relapses. A high proportion of inactive L cases treated only with sulfone have relapsed. 10-16
Quagliato, Bechelli and Marques15 observed in inactive L patients still on sulfone therapy that up to 3 years after bacterial negativity and clinical inactivity the number of relapses was small and increased substantially after 5, 9.5 or more years up to 30%.
The Marchoux Chemotherapy Study Group17 studied the frequency of relapses in MB patients after stopping treatment with rifampin-containing combined regimens. It was confirmed that relapses occurred late after completion of treatment (ultra-short courses, 3 of 6 months duration, 1 of 12 months, 2 of 24 months). A great majority of relapses described in the paper have been confirmed by mouse foot pad inoculation. Among 437 eligible patients, 384 have been seen at least once later than 12 months after completing treatment. Out of these 384, 255 had not received antileprosy treatment previously and 129 (33.6%) had received treatment.
The overall relapse rale among 384 patients was 17.7%. Among patients followed up for more than 1 year, relapse was 17.1% after 7 years and 25% after 10 years, higher among those with a BI > 5 (21.3%). "The risk of relapse was significantly smaller among those which achieved smear negativity (p < 0.01). However achievement of smear negativity does not guarantee that the patients will not relapse." ". . . Because of the late appearance of relapse patients should be followed-up for at least 7 to 10 years after completing chemotherapy. Only after a long period of follow-up may one draw final conclusions with respect to the efficacy of any RMP-containing combined regimen, including WHO/MDT. . . . Therefore the relative low relapse among patients treated with WHO/MDT must be interpreted with caution pending longer followup. Because millions of leprosy patients have been or currently are being treated with the WHO/MDT regimen, we strongly suggest that the MB patients, especially those whose BI is still high after completion of treatment, be closely followed-up."
During the last 15 years Pattyn18 and coworkers have been involved in studies on short MDT regimens for the treatment of MB leprosy: the duration of treatment varied from 104 weeks to 52, 34, 13 and 4 weeks. All regimens were triple-drug regimens, except for one double-drug regimen and one with four drugs. "Overall, relapses occur more frequently among patients with a higher BI at the start of the treatment. . . . The WHO regimen applied to previously untreated, highly bacilliferous patients . . . is followed by a cumulative rate of 10% at 5 years."
He concluded that "to evaluate treatment regimens in MB leprosy it is necessary to follow-up patients until 9-10 years after the end of treatment . . ."; the risk of relapses increases with time. Furthermore, "the absence of relapse during the first 3 years does not exclude high relapse rates later on." Regarding the dormant bacilli he adds: "For the time being it seems logical to administer to those patients who have been treated in the past with whatever combined regimens and who are 'inactive,' a short course of a few days of a bactericidal drug such as RMP once a year, for 10 years, after the end of the treatment to eliminate any possible bursts of dormant bacilli."
In the 14th International Congress of Leprosy (1993), the Workshop on Chemotherapy "suggested the fixed duration regimen may prove to be inadequate in previously untreated LL patients with a high bacterial load, and counselled caution in the widespread adoption of 2 years fixed duration treatment of WHO/MDT until further data are available. It also noted that many relapses are occurring late and, therefore, five years' post-treatment follow-up appears to be very inadequate, 8-10 years being the minimum required."
From the findings mentioned, it appears that a significant proportion of MB patients are expected to have a late relapse after stopping the 2-year WHO/MDT. Therefore to reduce to a minimum the relapse rate and the risk of a late recrudescence of the endemic, MB cases should be treated until the subsidence of all signs of activity and achievement of smear negativity. The minimum period of regular treatment should be extended from 2 to 3-5 years, according to the severity of the disease and bacterial load. The post-treatment follow up should be of about 10 years.
WHO/MDT in PB leprosy (6-month treatment). In PB leprosy spontaneous regression of the skin lesions occurs in a high proportion of cases, from 53% to 88%8,9 Although only a certain proportion of PB cases would really need MDT, the impossibility of identifying them -unless lepromin testing is performed -and the risk of disabilities render the treatment of all of them obligatory.
According to Pattyn,18 to be effective in PB leprosy a dose of RMP should be associated with several other doses of RMP, either daily for 6 days or weekly for 8-12 weeks or monthly for 6 months, or with daily DDS for 1 year.
From the studies of Nadkarni, Grugni and Kini19 and Katoch, et al. 20 it appears that PB patients treated for 6 months by WHO/ MDT had a lower proportion of inactivation and a higher percentage of deterioration in contrast to a higher proportion of inactivation and very low rate of deterioration with WHO/MDT (6 months) followed by DDS (6 months). Besides, unfavorable evolution occurred mainly in the first 2-3 years of follow up. These findings lead to the conclusion that WHO/MDT should be carried out at least for 1 year and treatment stopped only when there is no sign of activity. A follow-up period of 2-3 years is advisable mainly for patients who initially had three or more lesions.
Incidence. To achieve a real decline of the endemic it is essential to get a significant and lasting decrease of the incidence. This takes a long time, and is not accomplished unless the rate of MB cases and, consequently, the load of infectiousness is substantially reduced. Otherwise, after an apparent success of the campaign and a possible attenuation of the control measures, a reactivation of the endemic would occur in the long term, as observed with tuberculosis in recent years.
According to Noordeen,2 "Although MDT can drastically reduce prevalence of existing cases, its capacity to reduce incidence of new cases, at least in the first years of MDT implementation, is rather limited."
The decline of incidence takes a relatively long term, as noted in an excellent control project in Central African Republic. Nebout21 reported the data regarding prevalence and incidence from 1958 to 1980, before the use of MDT. The population was 1,250,000 inhabitants in 1962 and 2,050,000 in 1981. The number of registered cases was 65,388 in 1958 (66 per thousand), the highest in the world; in 1980 the rate was 5.7 per thousand. Almost 50% of the patients were regularly treated (> 75% of the dose prescribed was taken), 27.4% intake of 40%-74% and 25.6% less than 40%. The rate of new cases per thousand was 2.37 in 1960, 0.54 in 1970, 0.34 in 1978, 0.33 in 1979, and 0.37 in 1980. However, the incidence rates per 1000 of L cases have slightly increased: 0.025 (1960), 0.028 (1970), 0.032 (1978), 0.035 (1979), and 0.035 (1980).
WHO/MDT side effects. Taking into account the data concerning MDT in 3507 patients in the period between 1986 to 1988, Ramu2 2 stated that side effects occur only occasionally. However, a few cases had hepatotoxicity, oliguria, acute renal failure, flu syndrome, exfoliative dermatitis, methemoglobinemia, shock, night blindness, lymphadenopathy. Hemolysis occurred in 17 cases; red and black pigmentation and ichthyosis were common.
Our University Hospital (Hospital das Clínicas) has received some patients with very serious adverse reactions to MDT: hepatotoxicity, jaundice, renal failure. One of them died, and the autopsy showed liver necrosis and hemorrhage, disseminated intravascular coagulation and gastric erosions.
It is important that staff get adequate training to recognize MDT side effects and to take relevant action. Hospital facilities are required to attend the patients with serious adverse reactions. It seems that these often occur at the third intake of rifampin.
2. Difficulties in implementing MDT and control programs
(This topic is interlinked with section 3. Socio-economic, cultural and other factors in leprosy endemic areas (discussed later).)
The difficulties may be easily evaluated, considering the table from LEP News.1 The number of registered cases was 3,087,788, including 584,412 new cases. The estimated number of leprosy cases worldwide in 1991 was 5.5 million. The number of cases on MDT was 1,295,640, roughly 42% of the registered cases. This means that in spite of the WHO, governments and nongovernmental organizations' praiseworthy efforts, 58% of registered patients were not yet receiving MDT nor were the estimated 2,400,000 undiagnosed cases.
Marked progress was achieved in 1992.23 "The proportion of registered cases being treated with MDT at the time of this report is about 49% and the cumulative MDT coverage since this strategy was introduced has reached 22%. More than 5.3 million were or are being treated with MDT. . . ."
From a WHO epidemiological study in an efficient control project in Myanmar24 it appears that 13% of L cases, 48% of B, 43% of I and 54% of T cases were undiagnosed. The PB patients are less important in spreading the disease but 5%- 10% of untreated I cases could evolve to L or B and become contagious. Also a certain proportion of undiagnosed and untreated I and T patients could develop disabilities.
Before WHO/MDT was introduced, the findings of the WHO Leprosy Advisory Team in Africa (northern Nigeria; north, central and south Cameroon) in a random sample survey25 showed that even in countries with fairly good case-finding programs new cases amounting to 75% of the number of registered cases were detected.
The difficulties for implementing WHO/ MDT and for the development and success of control programs are related to characteristics of the disease itself; poor health infrastructure in many countries, adequate in only a few; limitations of treatment and preventive methods; education and cultural background; socioeconomic and other factors (section 3). Furthermore, in the scale of priorities leprosy comes after several diseases, especially AIDS, malaria, tuberculosis, trypanosomiasis, cholera, schistosomiasis, STD and others, depending on the countries and areas.
According to Nakajima,26 WHO Director General, "AIDS is rapidly becoming a most serious threat to human existence. WHO estimates that 8.10 million adults may currently be infected with the human immunodeficiency virus. More than half of these will develop AIDS within ten years and most will die. . . . The global malaria situation has become critical in recent years. . . . The disease is endemic in some 100 countries. .. . In West Africa 18 million people are infected with onchocerciasis. . . . Widespread malnutrition and improper nutritional practices are evident, even in areas where food is plentiful. . . ."
In fact, especially where leprosy is highly prevalent, the health service has to deal also with diseases of higher endemicity and/or high mortality rates.
Funds available are very often limited and frequently full advantage of resources is not taken because of inadequate planning and programming. It is difficult or impossible to increase leprosy budgets proportionately, and the speed of work is thus reduced, to the great detriment of the development of the general program.
It should be added that in many endemic areas there is a shortage of doctors or most of them are not interested in working in leprosy control projects. Salaries are usually low or not considered high enough to encourage staff to devote themselves fully to their work. Furthermore, health campaigns have been hampered by the appointment of personnel, even in supervisory posts, who lacked the necessary technical qualifications.
The above factors have been analyzed in detail by Bechelli27,2 8 and Martinez and Bechelli.8
Millan29 reported that 10 years after WHO had recommended MDT, this could be applied only to a minority of patients in Africa. However certain countries, members of OCCGE, have achieved considerable progress in the last 2 years to improve the MDT coverage. This varies from 8.4% in Mali to 97% in Benin. With MDT the prevalence rate in eight OCCGE countries dropped from 4.97% in 1982 to 1.09% in 1990. However, it seems that in the same period the detection rate continued to be between 1.2% and 1.8%.
Millan29 also stresses that ". . . the application constraints of MDT raise very difficult operational problems that hinder its generalization. To be effective the MDT coverage should be preceded, as done in Benin and Senegal, by an intense preparation implying the reorganization of duties, actualization of archives and training of relevant staff."
In the investigation by the Marchoux Chemotherapy Study Group1 7 regarding relapses, 50% of their 324 patients had been lost to follow up 5 years after completion of treatment. It is probable that in routine campaign work the proportion would he higher.
In India, according to Ramu22 ". . . irregularity in intake of self administered clofazimine has been occurring due to poor patient compliance as well as irregular drug supply ... . Patients who are taking self administered drugs irregularly would be on rifampicin monotherapy. There is a great danger of emergence of rifampicin resistance in them and rifampicin resistance has already been documented in 39 cases relapsing under rifampicin monotherapy." (Grosset, et al. 1989)
Languillon30 considers that MDT is "relatively possible" in urban areas but not in rural zones. In the French-speaking countries good roads among villages allow the mobile teams to treat and control the patients. However, after 6-8 months of treatment they improve and become irregular or stop the drugs. This does not happen in the pilot areas where MDT has a special surveillance. It is also noted that during the period of agricultural activities patients do not attend regularly. Languillon mentions the ILEP programs, 620 in 88 countries: out of 831,000 patients only 32% were receiving MDT.
When only dapsone was used Laviron31 reported that in French-speaking Africa, 10% to 60% of the patients were under regular treatment, around 60% were not yet detected, and about 50% were out ofconlrol ("lost sight of").
With WHO/MDT what is the proportion of MB cases that in fact have taken the drugs irregularly over 2 years? Probably a significant proportion of them are taking self-administered drugs irregularly. For decades this has been a constant difficulty in leprosy projects and also in the control of other chronic diseases. Thus the efficacy of treatment would be reduced while the risk of relapse would be increased.
3. Socioeconomic, cultural, education, demographic, political and related factors in leprosy-endemic areas
There is an intimate relationship between the health situation and the economic level which, in its turn, depends on very different ecological, demographic, historical, cultural and sociological factors. A leprosy endemic is present in developing countries or areas. In the highly industrialized countries leprosy had disappeared or is practically absent, even before the advent of sulfones. In addition, in these countries "imported" cases have not disseminated the disease. This spreads where the inhabitants face unfavorable socioeconomic and related conditions: penury, hunger, malnutrition, high infantile mortality, unemployment, low minimal salary, inflation, recession, poor housing, slums ("favelas"), promiscuity, lack of hygiene, illiteracy, no family planning, ecological degradation, corruption, political instability, rebellion, internal war, etc.
In an editorial in the World Health Forum, the WHO Director General Nakajima26 analyzes health and development in the 1990s: "In spite of improvements in the world health situation, the disparity between developed and developing countries and even between population groups in some countries remains great. The high rate of avoidable maternal mortality in many developing countries, and the difference in the expectancy between the rich and the poor, are unacceptable. . . . Notwithstanding some social indicators show that even at low-income levels, impressive human development can be achieved. Yet we still have a long way to go to realize the goal of health for all people, everywhere. . . . The issue is how to stem the rising tide of socio-economic conditions that deprive millions of fellow human beings of the basic conditions for health, and for leading a decent and productive life. . . . Accompanied by the lack of economic growth, rising unemployment, diminishing expenditure on other healthrelated sectors (such as education, water supply and sanitation) and the natural disasters that beset certain countries and regions, this means that millions of people remain critically vulnerable at the start of the new decade. . . ."
The above facts and considerations give the picture of the difficulties that WHO and governments are facing and have to overcome in order to reach the objectives of different health campaigns, including the elimination goal of leprosy by the year 2000. Tuberculosis may be mentioned as an example. With BCG and the advent of new drugs since the 1950s, the disease was under control. However, due to adverse conditions, including the spreading of AIDS, there has been a significant increase in the incidence and number of deaths.32
Taking into account what was considered in sections 1, 2 and 3, it does not seem possible to achieve the global elimination of leprosy as a public health problem by the year 2000. In the next 7 years it will be extremely difficult, if not impossible, for all of the endemic countries to reach the desirable socioeconomic level and overcome all the unfavorable conditions. The alternative would be the availability in the very near future of an efficient vaccine and/or a very effective and easily administered drug, as much as possible cheap and free from serious side effects, capable of reducing to a very short period the duration of treatment.
Even if the elimination goal established by the World Health Assembly in May 1991 is not reached, the praiseworthy efforts of WHO, governments, nongovernmental organizations and specialists shall be of great benefit to countries and populations.
In light of the data presented, the following conclusions can be drawn:
• In randomized trials MDT regimens were not significantly superior to DDS alone. Furthermore, a significant proportion of late relapses have been observed in MB patients. The present findings do not confirm that the drastic reduction of leprosy prevalence, from 10-12 million to 5.5 million since 1982, was due to the success of WHO/MDT. When this was adopted the duration of treatment was considerably shortened. The anticipated release from control of all treated cases caused, automatically, a considerable reduction of prevalence.
• Late relapses have been observed in a significant proportion of MB patients, especially those with a high BI when stopping MDT. To reduce to a minimum the relapse rate and the risk of recrudescence of the endemic, MB cases should be treated until the subsidence of all signs of activity and the achievement of smear negativity. The minimum period of regular treatment should, therefore, be extended from 2 to 3 - 5 years, according to the severity of the disease and bacterial load. The post-treatment follow up should be about 10 years.
• Unfavorable evolution was more often observed in PB patients submitted to only 6 months of WHO/MDT. Thus, it is necessary to carry out regular treatment with MDT for at least 1 year, and the treatment should be stopped only when there is no sign of activity. A follow up of 2-3 years is then required, mainly for patients who initially had three or more lesions.
• Considerable progress has been achieved in the therapy of leprosy and its control in the last 50 years, and the WHO/ MDT and other MDT regimens are recommended because of the risk of the development of resistant strains of M. leprae with monotherapy. However, the present situation regarding the control of leprosy with WHO/MDT is similar to that of syphilis treated by arsenical + bismuth + mercury until 1943 when penicillin was introduced. So far there is not an ideal single drug or combined regimen for leprosy therapy.
• Many unfavorable conditions in endemic areas hinder the implementation of WHO/MDT and the control of leprosy and other diseases.
• In view of the above, it does not seem possible to achieve the global elimination of leprosy as a public health problem by the year 2000, unless an ideal drug and/or vaccine becomes available in the very near future. With BCG and effective drugs, tuberculosis was under control for a long period but, in recent years, its morbidity and number of deaths have increased significantly. Syphilis has not yet been eradicated in half a century in spite of the effective and simple treatment with penicillin.
It is expected that the endeavors of WHO and institutions and scientists concerned with the problem may lead, in the near future, to the discovery of the ideal drug and/ or vaccine, the only way of controlling leprosy in a relatively short period, as yaws was practically eradicated and smallpox eradicated, in the most adverse conditions, in two remarkable WHO programs.
- Luis Marino Bechelli, M.D., Ph.D.
Professor of Dermatology
Faculdade de Medicina de Ribeirão Preto
University of São Paulo, Brazil
Former Chief Medical Officer
Leprosy Unit
World Health Organization
Geneva, Switzerland
Acknowledgment. The author wishes to thank Dr. Paulo M. G. Pagnano for his comments on the manuscript, Drs. Ana M. F. Rosclino and Ana N. Almeida for the graphic, J. C. Mazotti Filho for the data on side effects, and Miss Marisa T. Hayashi and Mrs. Regina H. B. Benatim, secretaries of the Department of Internal Medicine.
1. WHO LEP News 1(1992).
2. Noordeen, S. K. Elimination of leprosy as public health problem. Indian J. Lepr. 63(1991)401-409.
3. Faget, G. H., Pogge, R. C, Johansen, F. A., Dinan, J. F., Prejean, B. M. and Eccles, C. G. The promin treatment of leprosy; progress report. Public Health 58(1943)1729-1741.
4. Dietrich, M. J., Rangaraj, J., Ganapati, R., Devanbu, V., Jayakumar, T., Chiang, T., Meyers, W. M. and Gaus, W. Combination therapy/vs monotherapy in BL and LL patient: a prospective randomized multicenter study. (Abstract) Int. J. Lepr. 57 Suppl. (1989)425-426.
5. Irudayaraj, P. P. and Aschhoff, M. Assessment criteria and multidrug therapy. (Abstract) Int. J. Lepr. 57 Suppl. (1989)426.
6. Subcommittee on Clinical Trials, Scientific Working Group on the Chemotherapy of Leprosy (THELEP). Response of THELEP trial patients to combined drug regimens. (Abstract) Int. J. Lepr. 57 Suppl. ( 1989)
7. Martinez, D. V. and Bechelli, L. M. Prospects of controlling leprosy in tropical Africa. Acta Leprol. 66-67(1977)19-25.
8. Lara, C. B. and Nolasco, J. O. Self-healing, or abortive and residual forms of leprosy and their probable significance. Int. J. Lepr. 24(1956)245-263.
9. Dharmendra's communication to WHO, 25 May 1964; apud Bechelli and Martinez, WHO Guide to Leprosy Control, 1966, 12.
10. Erickson, P. T. Relapse following apparent arrest of leprosy by sulfone therapy. Publ. Hlth. Rept. 65(1950)1147-1157. Reprinted in Int. J. Lepr. 19(1951)63-74.
11. Lowe, J. The late results of sulphone treatment of leprosy in east Nigeria. Lepr. Rev. 25(1954)113-124.
12. Rodriguez, N. J. Relapses after sulfone therapy in leprosy of the lepromatous type. Int. J. Lepr. 26(1958)305-312.
13. Price, R. B. Relapse of leprosy in American Samoa. Amer. J. Trop. Med. Hyg. 8(1959)358-363; apud Int. J. Lepr. 27(1959)396.
14. Quagliato, R., Bechelli, L. M. and Marques, R. M. Bacterial negatively and reactivation (relapse) of lepromatous out patients under sulfone treatment. Int. J. Lepr. 38(1970)250-263.
15. Noordeen, S. K. Relapse in lepromatous leprosy. Lepr. Rev. 42(1971)43-48.
16. Jacobson, R. R. and Trautman, J. R. The treatment of leprosy with sulfones. Int. J. Lepr. 10(1971)726-737.
17. Blanc, L., Bobin, P., Daumeric, D., Discamps, G., Grosset, J., Grossetete, G., Husser, J. A., Jamet, P., Nebout, M., Pattyn, S. and Traore, I. Relapses in multibacillary leprosy patients after stopping treatment with rifampin-containing combined regimens. Int. J. Lepr. 60(1992)525-535.
18. Pattyn, S. R. Search for effective short-course regimens for the treatment of leprosy. Int. J. Lepr. 61(1993)76-81.
19. Nadkarni, N. I., Grugni, A. and Kini, M. S. Fixed duration MDT in paucibacillary leprosy (classic and modified). Int. J. Lepr. 61(1993)25-28.
20. Katoch, K., Ramanathan, U., Natarajan, M., Bagga, A. J., Bathia, A. S., Saxena, R. K. and Ramu, G. Relapse in paucibacillary patients after treatment with three short term regimens containing rifampin. Int. J. Lepr. 57(1989)458-464.
21. Nebout, M. La lutte anti-lepreuse en R.C.A. Resultats obtenus. Acta Leprol. 86-87(1982)15-22.
22. Ramu, G. Problems of multidrug therapy. Indian J. Lepr. 63(1991)435-445.
23. WHO Weekly Epidemiological Record 68(1993)181-186.
24. Bechelli, L. M., Gallego, G. P., Mg MgGyi, Uemura, K., Sundaresan, T., Tamondong, C, Martinez, D. V. and Walter, J. Some epidemiological data on leprosy collected in a mass survey in Burma. Bull. WHO 48(1973)335-344.
25. Bechelli, L. M., Martinez Dominguez, V. and Patwary, K. M. WHO epidemiological random sample surveys of leprosy in northern Nigeria (Katsina), Cameroon and Thailand (Khon Kaen). Int. J. Lepr. 34(1966)223-241.
26. Nakajima, H. Health and development in the 1990s. World Health Forum 11(1990)355-357
27. Bechelli, L. M. Advances in leprosy control in the last 100 years. Int. J. Lepr. 41(1973)285-297.
28. Bechelli, L M. Futurology of leprosy. Aussatz. Lepra. Hansen-Krankheit, Teill II. Herausgegeben von J. H. Wolf; Würzburg, 1986, 409-414.
29. Millan, J. Le VIIIe Congrès des Léprologues de Langue Française. Acta Leprol. 8(1992)1-3.
30. Languillon, J. Polychimiotherapie de la lèpre en zone rurale. Acta Leprol. 6(1988)67-71.
31. Laviron, P. Lèpre; rapport sur un séminaire (Kampala), Bureau Régional de l'Afrique. AFRO 0218, 1970.
32. WHO Working Group Meeting. Tuberculosis research and development. WHO/TB/91.162.
Reprint requests to Dr. L. M. Bechelli, Rua Prudente de Morais 767/111, 14015-100 Ribeirão Preto, SP, Brazil.