- Volume 62 , Number 1
- Page: 10–23
Incidence rates of leprosy in Karonga District, Northern Malawi: patterns by age, sex, BCG status and classification
ABSTRACT
This paper describes incidence rates by age, sex, prior BCG status and classification in Karonga District, northern Malawi. New cases (489) were identified among 83,500 individuals followed for an average of 5 years (1.12 cases per 1000 person years). Only 29 (6%) of the incident cases were multibacillary. Incidence rates generally were higher among females than males, and increased steadily with age. Although the highest incidence rates of disease were recorded among young adults without BCG scars (males 15- 19; females 20-24), these peaks were less dramatic than those reported among young adults in The Philippines and Norway. In the absence of historical data and data on infection status, it is not possible to assess to what extent these peaks may reflect either greater exposure or greater susceptibility to disease among adolescents or young adults.The incidence rates of leprosy among individuals with a prior recorded BCG scar were approximately half those of individuals lacking a scar, at all ages. Since BCG had been introduced into this population only during the 1970s, this provides strong evidence for the effectiveness of BCG when given to adults. It was estimated that past vaccination of approximately 40% of the district population had reduced the overall incidence rate of leprosy by 18%, and that this impact would increase with aging of the vaccinated cohorts.
A retrospective examination of the detailed records of initial examinations revealed that 62 (13%) of the incidence cases were recorded as having skin hypopigmentation or blemishes, at the site of subsequent confirmed leprosy lesions, several months or years before they were suspected of having leprosy. The nonspecificity of these lesions, some of which were probably attributable to Mycobacterium leprae infection, highlights the difficulty of diagnosing leprosy in its earliest forms.
RÉSUMÉ
Cet article décrit les taux d'incidence par âge, sexe, statut antérieur vis-à-vis du BCG et la classification, dans le District de Karonga, dans le nord du Malawi. Des nouveaux cas (489) ont été identifiés parmi 83.500 personnes suivies pendant une moyenne de 5 ans ( 1, 12 cas pour 1000 personnes-années). Seulement 29 (6%) des cas incidents étaient multibacillaircs). Les taux d'incidence étaient généralement plus élevés chez les femmes que chez les hommes, et augmentaient régulièrement avec l'âge. Bien que les taux d'incidence les plus élevés de la maladie étaient enregistrés parmi des adultes jeunes sans cicatrice de BCG (hommes de 15 à 19 ans; femmes de 20 à 24 ans), ces pics étaient moins spectaculaires que ceux rapportés chez les jeunes adultes aux Philippines et en Norvège. En l'absence de données historiques et de données relatives au statut vis-à-vis de l'infection, il n'est pas possible d'évaluer dans quelle mesure ces pics peuvent refléter soit une plus grande exposition, soit une plus grande suseptibilité à la maladie parmi les adolescents ou les jeunes adultes.Les taux d'incidence de la lèpre parmi des personnes présentant auparavant une cicatrice de BCG étaient approximativement la moitié de ceux des individus qui n'avaient pas cette cicatrice, et ceci à tous les âges. Comme le BCG a été introduit dans cette population seulement durant les années 1970, ceci procure un puissant argument en faveur de l'efficacité du BCG quand il est administré à des adultes. Il a été estimé que la vaccination antérieure d'approximativement 40% de la population du District a réduit le taux global d'incidence de 18%, et cet impact devrait augmenter avec le vieillissement des cohortes vaccinées.
Un examen rétrospectif des enregistrements détaillés des examens initiaux a révélé que 62 (13%) des cas incidents avaient été notés comme ayant une hypopigmentation ou des défauts cutanés à l'endroit où, par la suite, des lésions lépreuses ont été confirmées, et ceci plusieurs mois ou années avant qu'ils n'aient été suspectés de lèpre. La non-spécificité des ces lésions, parmi lesquelles certaines étaient probablement dues à l'infection par Mycobacterium leprae met en lumière la difficulté de diagnostiquer la lèpre dans ses formes les plus précoces.
RESUMEN
Este articulo describe la incidencia de lepra por edad y sexo, los antecedentes de la vacunación con BCG, y la clasificación de la enfermedad en el Distrito de Karonga en Malawi del Norte. En un periodo de 5 años se identificaron 489 casos nuevos entre 83,500 individuos (1.12 casos por 1000 personas/años). Sólo 29 (6%) de los casos incidentes fueron multibacilares. La incidencia fue generalmente mayor entre las mujeres que entre los hombres y aumentó en forma sostenida con la edad. Aunque las mayores frecuencias de incidencia de la enfermedad oceurrieron entre los adultos jóvenes sin cicatrices de BCG (hombres 15-19, mujeres 20-24), estas cifras fueron menos dramáticas que las reportadas para los adultos jóvenes de Noruega o Las Filipinas. En ausencia de los datos sobre antecedentes y sobre el estado de la infección no es posible establecer si estas frecuencias de infección reflejan un mayor grado de exposición o una mayor susceptibilidad a la enfermedad entre los adolescentes o los adultos jóvenes de Malawi. El grado de incidencia de la lepra entre los individuos con una cicatriz previa de BCG fue de aproximadamente la mitad de la incidencia observada en los individuos sin cicatriz a todas las edades. Puesto que la vacunación con BCG fue introducida en esta población durante los años 1970s, los resultados podrían ser una fuerte evidencia de la efectividad del BCG aplicado en adultos. Se calculó que la pasada vacunación de aproximadamente el 40% de la población de este Distrito ha reducido la incidencia global de la lepra en un 18%, y que este impacto podría incrementarse con el incremento en edad de la pobación vacunada. El examen retrospectivo de los estudios iniciales reveló que 62 de los casos incidentes (13%) fueron originalmente registrados como casos con hipopigmentación o con moretones de la piel en el sitio donde varios meses o años después aparecieron las lesiones de la lepra. La inespecificidad de tales lesiones, algunas de las cuales fueron probablemente atribuíbles al M. leprae , subrayan la dificultad del diagnóstico de la lepra en sus formas más tempranas.Incidence data are of particular value for the prediction of trends and for the identification of risk factors. One of the classic studies of disease incidence was that by Doull on leprosy in The Philippines published 50 years ago (2). Subsequent published reports relevant to leprosy are those based on BCG trials in Uganda (17), Papua New Guinea (1) and Burma (10), and on repeated population surveys in South India, particularly Karigiri (16) and Polambakkam (18). In addition, because of its thoroughness, the Norwegian National Leprosy Register has provided incidence rate data (8). Only the original Philippine study has presented detailed data on incidence rates by age, sex and clinical type.
The main findings from these studies may be summarized as follows. Leprosy incidence rates rarely exceed 2 per 1000 per year, except among young household contacts (3). In many populations (The Philippines, South India, Norway), incidence in males has exceeded that in females, at least among adults. Cases in children below 5 years of age are rare. Age patterns of leprosy incidence differ between populations and may be difficult to interpret, in part because of the long and variable incubation period between exposure to infection and ultimate onset/diagnosis of disease. Peak incidence rates were reported among young adults in Doull's historical family studies in The Philippines (2) and in Burma (10), and in successive birth cohorts in the Norway leprosy register data (8). However, peak incidence rates were among older adults in the latter years of the Norwegian epidemic, a pattern which was interpreted to reflect a residue of infections contracted years or decades in the past, when transmission of Mycobacterium leprae was more frequent in the population. Thus age patterns of leprosy incidence at any given time may reflect past as well as contemporary risks of M. leprae infection, and also trends in other risk factors which may be associated with the manifestation of clinical disease among infected individuals. All studies have shown that multibacillary disease is exceedingly rare among children, and that the proportion multibacillary among new cases increases with age. This has been taken to reflect a longer incubation period of multibacillary compared to paucibacillary disease (3).
In addition to age and sex, the chief risk factors for leprosy incidence revealed in these studies are (the absence of) a BCG vaccination (7) and household contact with a known leprosy case (3). Several other factors have been suggested, such as household crowding and poor nutrition, but these have been difficult to establish, perhaps because they are intimately correlated with many other characteristics of the poverty complex (3).
The only published population-based data on incidence rates of leprosy in Africa are those derived from follow up of participants in a BCG vaccine trial initiated in 1960 among children 0-15 years of age, who were either close relatives or household contacts of known leprosy patients in the Teso District of Uganda (17).
This paper presents detailed data on incidence rates of leprosy in the population of the Karonga District, northern Malawi, during the decade 1980-1990. The data were drawn from the Lepra Evaluation Project (LEP), a large epidemiological study funded primarily by LEPRA (British Leprosy Relief Association).
MATERIALS AND METHODS
The study population and methods of the LEP have been described in detail elsewhere (13, 14). Incidence-related data described in this paper were collected during two total population surveys, one (LEP1) carried out 1980-1985 and the second (LEP2) carried out 1986-1989 in Karonga District, northern Malawi. The second survey served also as the recruitment phase for a vaccine trial, the "Karonga Prevention Trial" (KPT). The population in Karonga District is predominantly rural, dependent upon subsistence agriculture and fish from Lake Malawi for food and livelihood.
The survey work was carried out by field teams which moved across the district visiting each household. Basic identifying information collected by interviewers on all individuals included name, sex, date of birth (ascertained with the help of local events calendars), names of parents, village and geographic coordinates of the household (read off scaled aerial photographs).
All individuals were examined by trained paramedical workers (Leprosy Control Assistants = LCAs). Examination results were recorded on "General Examination Forms, "which included a body outline. Each examination was independent since results of previous examinations were not available in the field. Most examinations were "complete," excluding only the groin area, and any areas not examined were indicated on the form. The supra-orbital, greater auricular, upper and superficial radial, upper and lower ulnar, lateral popliteal (common peroneal) and superficial peroneal nerves were palpated routinely. The BCG scar status was recorded (as "present," "absent" or "doubtful") at each examination. Birthmarks, rashes, fungal infections, scars and other unusual features (amputations, extra digits, blindness, etc.) were also recorded.
A more extensive "Detailed Examination Form" was filled in for individuals with any lesions considered to be suspicious of leprosy. Information recorded included complete description and charting of all suspect lesions and the results of sensory loss testing (diminished pain, loss of thcrmosensation and anesthesia to light touch). All suspects were reviewed by a medical officer (usually J MP) who obtained a biopsy (4-mm punch or, if indicated, split nerve). Biopsies were sent to Dr. A. C. McDougall (until 1987) or Dr. S. B. Lucas (thereafter) for processing, and reported according to a standardized protocol as described elsewhere (4, 11). Slitskin smears were obtained from all individuals in whom there was a suspicion of multibacillary leprosy. The decision to include an individual in the analysis as a case of leprosy was determined by consideration of all historical, clinical, bacteriological and histopathological information according to an algorithm designed for this purpose (l2). Analyses presented here include cases in the N ("narrow" = most specific) and M ("middle" = probable) categories (l2). Cases are classified as multibacillary (MB) if they ever had a bacterial index (BI) > 2 on slit-skin smear, a BI > 2 on either skin or split-nerve biopsy or a histopathological classification of BB, BL or LL leprosy; otherwise they were called paucibacillary (PB).
All data were checked and coded at project headquarters and entered on computers for ultimate analysis in London. Analyses reported here were carried out on an IBM RS/6000 using SAS and EGRET software.
Incidence rates described in this paper are based upon analysis of data on individuals seen at least twice during the course of LEP 1 and/or LEP2, with an interval of at least 30 days between the initial and last examinations. Individuals were excluded from the analysis if there was a history of leprosy, or any suspicion of the disease as recorded by the medical officer, at the time of his or her initial examination. The follow-up period for all individuals continued until the first of the following dates: a) when leprosy was first suspected; b) receipt of a KPT vaccine, or c) the last recorded examination prior to 1 January 1990. In order to confirm the incidence status of cases, i.e., that the lesions actually appeared after the first examination by LEP project staff, the initial records of all new cases were reviewed (by JMP). Special note was taken of all those whose initial records included mention of a hypopigmcntcd spot or blemish at the site of a future leprosy lesion. Although these spots or blemishes originally had not been suspected to indicate leprosy, the appearance of a confirmed leprosy lesion at the same site in later years indicates that at least some of them were very early leprosy lesions. Separate analyses were carried out including and excluding these individuals from both the numerator and denominator of the incidence rate calculations.
For analysis purposes, the BCG scar status of each individual is based upon all pre1990 examinations up to the time of his or her recruitment into the KPT. Any individual with an inconsistent scar status record (i.e., in whom a scar was recorded as present at one examination and absent at another), or in whom the scar was ever recorded as "doubtful," is considered to have a doubtful ("?") scar.
Results are presented by contemporary age, not age at first examination. Thus an individual who was 10 years of age when first examined in 1980 may have contributed 5 years at risk to the 10-14 age group and 3 years at risk to the 15-20 age group before his or her last examination in 1988.
RESULTS
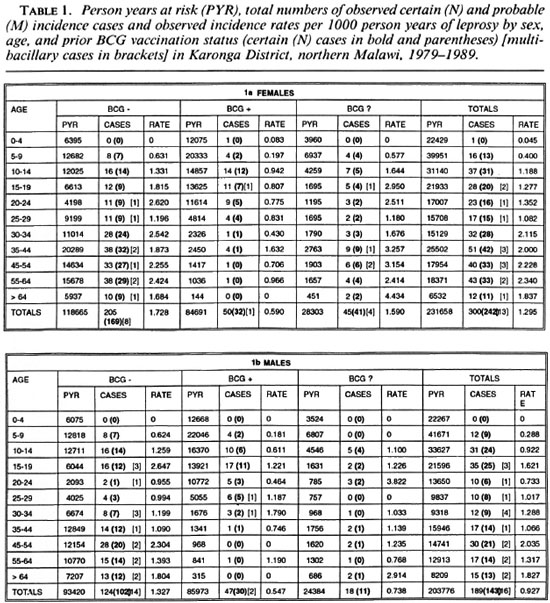
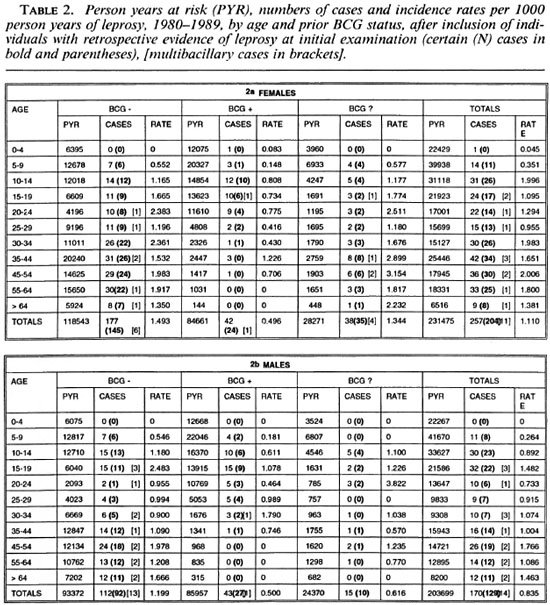
A total of 109,310 individuals were examined between 1979-1985 in LEP1 and considered at that time to have no evidence of past or present leprosy. Of these, 81,111 (72%) were examined during the LEP2 survey (1986-1989). In addition, 2389 individuals were examined more than once in one or the other survey, and hence provide data for incidence rate analysis. The distribution of person years at risk of all 83,500 persons included in these analyses is shown by age, sex and BCG scar status in Table 1. The decline in person years with age reflects the age structure of the population. In addition, the relative deficit of person years contributed by young adults, in particular males, reflects their tendency to leave the district to find work elsewhere. The majority of person years below 25 years of age were contributed by individuals with a prior BCG scar; whereas the opposite was true for older ages, reflecting the relatively recent introduction of BCG into this population (6).
The frequency distribution of follow-up periods for all 83,500 individuals in the incidence analysis is shown in Figure 1; 70,236 (84%) of the individuals were followed up for approximately 5 (between 4 and 6) years.
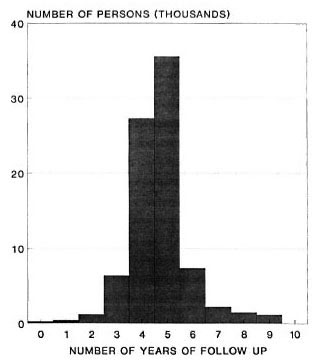
Fig. 1. Frequency distribution of follow-up periods among 83.500 individuals included in these analyses.
A total of 489 new leprosy cases was ascertained in this population during the follow-up period. Of the 489 cases, 385 (79%), including all 29 MB cases, were considered certain (N) leprosy. The cases are broken down by age, sex, BCG scar status, diagnostic certainty and paucibacillary-multibacillary classification in Table 1. The age distribution of total incident cases is shown in Figure 2. The crude leprosy incidence rate in this population was 489/435,434 = 1.12 per 1000 person years.
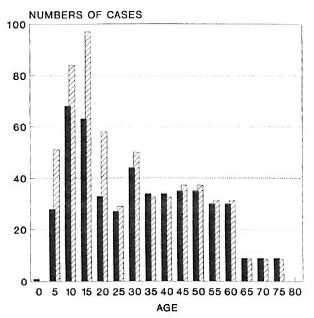
Fig. 2. Age distribution of the numbers of certain and probable incident leprosy cases observed  and expected
and expected  on the basis of rates in liCG scar negatives.
on the basis of rates in liCG scar negatives.
A review of the initial general examination forms revealed that 62 (13%) of the 489 apparent incidence cases had some record of a hypopigmented "spot," "blemish" or "birthmark" on a site where a confirmed leprosy lesion was later recognized. Table 2 shows incidence rate estimates calculated after exclusion of these 62 individuals from both the numerators and denominators (i.e., assuming they already had clinical leprosy at the time of the first survey). The overall crude incidence rate after excluding these individuals was 427/435,174 = 0.98 per 1000 person years. Subsequent figures are based on all 489 cases, without exclusions, as explained in the Discussion.
Age-specific rates are presented in Figures 3-6, showing successive stratification by sex (Fig. 4), sex and BCG status (Fig. 5), and classification (Figs. 6A and 6B). Adult ages are grouped as 15-24, 25-34, 35-54 and 55 plus in order to smooth age trends in the rates. Individuals with doubtful BCG scar status are omitted from Figure 5.
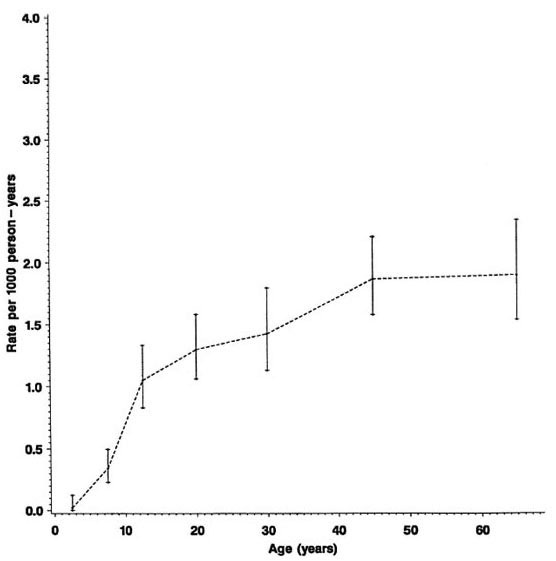
Fig. 3. Incidence rates of leprosy (N and M cases combined) by age, Karonga District, Malawi, 1980-1989.
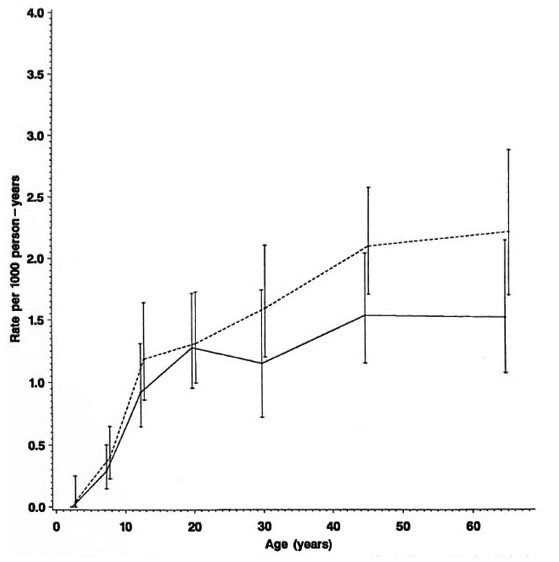
Fig. 4. Incidence rates of leprosy by age and sex (N and M cases combined), Karonga District, Malawi,1980-1989; 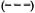 = females;
= females;  = males.
= males.
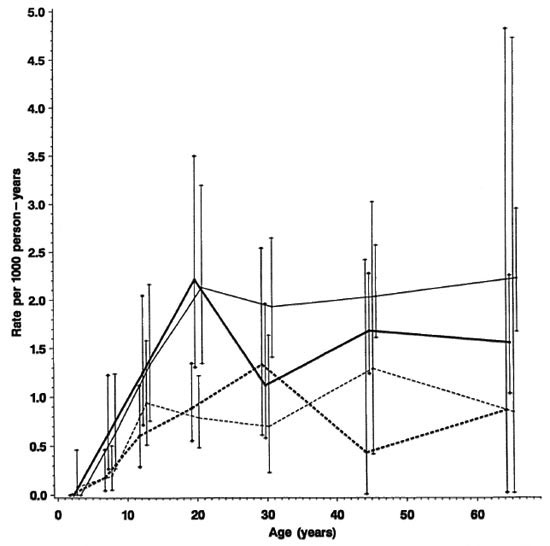
Fig. 5. Incidence rates of leprosy by age, sex and BCG scar status (N and M cases combined), Karonga Dis-trict, Malawi, 1980-1989;  = F scar positive;
= F scar positive;  = F scar negative:
= F scar negative:  = M scar positive:
= M scar positive:  =M scar negative.
=M scar negative.
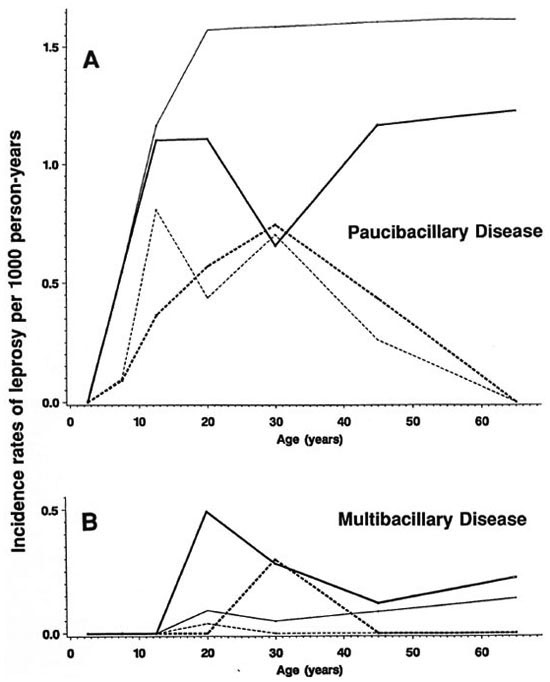
Fig. 6. Incidence rates of paucibacillary (A) and multibacillary (B) leprosy by age, sex and BCG scar status, Karonga District, Malawi, 1980-1989;  = F scar positive;
= F scar positive;  = F scar negative;
= F scar negative;  = M scar positive;
= M scar positive;  = M scar negative.
= M scar negative.
The overall rates rise progressively by age, approaching 2 per 1000 person years among older adults (Fig. 3). From Table 1 we see that the incidence rates were slightly lower for the 20-29 age group than for the 15-19 age group. This peak among teenagers/young adults is not evident when the data are age grouped as in the Figures.
Separation by sex shows that rates were in general higher among females (Fig. 4) except for a slight excess among males in the 15-19 year age group (Table 1). Further stratification by BCG scar status (Fig. 5) shows a consistently lower rate among those considered BCG "positive," with the exage, sex and BCG status reflect rates of PB ception of males aged 25-34. It is worth disease, which accounted for 460 of 489 noting the wide confidence limits for the (94%) of the incidence cases in this popurates in this age group, due to the relatively lation. A detailed breakdown of MB cases small numbers (Table 1). The difference in incidence rates between persons without and with a BCG scar reflects the protection imparted by BCG in this population (15), and shows that the effect is consistent in bothsexes and at all ages.
Stratification by disease classification (MB versus PB) shows that the overall trends by age, sex and BCG status reflect rates of PB disease, which accounted for 460 of 489 (94%) of the incidence cases in this population. A detailed breakdown of MB cases is difficult because of the small numbers involved and, thus, is presented in broader age groups (Figs. 6A and 6B). The incidence rates of MB disease were zero below age 15, were generally higher among male than female adults, and were appreciably loweramong those with than in those without a prior BCG scar.
Table 3 presents the percent reduction in leprosy risk associated with a BCG scar (= vaccine effectiveness) broken down by diagnostic certainty (N versus M cases), classification, and with or without the exclusion of cases whose lesions may have arisen prior to the initial examination. The protection was significantly greater against certain (N) than against probable (M) PB cases combined (60% versus 3%, p = 0.004), and marginally greater against certain (N) MB than against certain PB disease (76% versus 60%, p = 0.18). The point estimates of efficacy were lower against cases which were found (retrospectively) to have had a lesion at the start of the survey than in those whose lesion appeared after their first examination (17% versus 54%, p = 0.15).
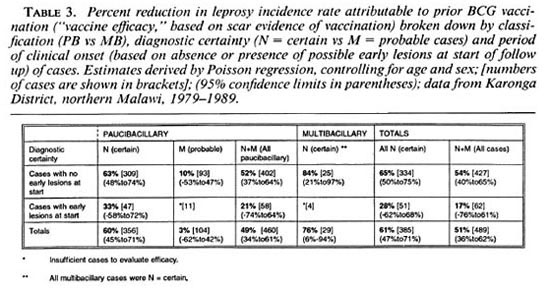
Figure 2 shows the numbers of leprosy cases expected in this population if the age-sex specific rates observed in BCG-negative individuals had applied to the entire population (i.e., numbers expected if there had been no BCG vaccination in the population).
DISCUSSION
The overall observed incidence rate of (certain and probable) leprosy in this population during the study period was 1.12 per 1000 person years. This is high by global standards. Indeed, the original selection of Karonga District for the LEP had been based upon indirect evidence that the disease was particularly common in this population. Only 29 (6%) of 489 newly identified cases were MB, and of these 29, four were MB only on the basis of a BI > 2 in a split-nerve biopsy.
Incidence rates reported here were based upon a 5-year follow-up period (Fig. 1). Given that some cases may self-heal, it is likely that higher rates would have been observed if the follow-up interval had been shorter (9).
Retrospective examination of the initial records of these 489 apparent incidence cases revealed that 62 (13%) had a skin blemish noted at the site where a leprosy lesion was later identified. It should be emphasized that the "lesions" on these individuals were thought only in retrospect to be possibly indicative of early leprosy since the individuals had not been classified as suspects by project staff when first seen. These 62 cases represent a unique observation insofar as we are not aware of any previous study to have recorded such meticulous notes of so large a number of individuals not considered to have leprosy, and then to have followed them up. We have not excluded them from subsequent descriptive analyses for the following reason: given that such lesions could only be recognized as early leprosy in retrospect, it is not possible to identify analogous individuals at the end of follow up and, thus, their exclusion from the analysis would have the effect of underestimating the true incidence rates on the population. It may be noted, however, that if the intention of this paper were a detailed analysis of risk factors acting at the time of or after the initial examinations, then such cases would best be excluded. This is illustrated in the higher protection afforded by BCG against lesions which truly had onset after the initial examinations.
Presentation of these incidence data by age, sex and BCG status illustrates that each of these factors is an important determinant of leprosy risk (Figs. 3-5).
Given the strong influence of BCG, and the fact that BCG scars were far more prevalent among younger than among older individuals (Table 1), the effect of age is best revealed among individuals with no history of BCG (Fig. 5). Among such individuals the incidence rate rose to almost 2 per 1000 by age 20 and then remained roughly stable for older age groups. There is some evidence for peaks among young adults (15-19 year old males, 20-24 year old females; see Tables 1 and 2, Fig. 5), but these may be due to chance and are, in any case, less dramatic than were seen in the Philippine or Norwegian data (2, 8). The absence of a clear peak among young adults, and the high incidence rates among older adults, may be interpreted in various ways. If incidence had been stable in the population during several decades prior to this study, then the age trends would reflect a combination of agespecific patterns of exposure, of susceptibility and of incubation period. Under the stable incidence assumption, the relatively high incidence rates in adults would suggest either that many individuals were not infected until late in life or, if infection occurred early in life, that incubation periods were very long, perhaps reflecting a reactivation process analogous to that in tuberculosis.
Alternatively, if incidence rates had been declining in the population for many years, the observed high incidence rates among older adults would be attributable to infections contracted many years previously when transmission of M. leprae was more intense than at the time of these surveys. There are no data from the past allowing us to discriminate between these explanations.
Again restricting consideration to those with no evidence of prior BCG, the incidence rates were higher among females than among males over 20 years of age, except for the oldest age group. Such a female excess is consistent with published evidence for higher prevalence among females in several populations in Africa, but contrasts with higher prevalence and incidence reported among males in studies from Asia. There is some evidence that the higher incidence in males in Asia is, in part, an artifact of differential ascertainment (3). To what extent the female excess in Africa might be due to greater exposure, or to greater susceptibility to disease among those who are infected, is unknown.
The data show no evidence for the BCG-associated reduction in risk to be lower at older than younger ages. This is surprising for two reasons. First, we have shown elsewhere that the validity of a scar as an indicator of past BCG vaccination declines with age (6). On this basis, we might expect the observed efficacy of BCG to be reduced in older individuals, if only because of increased misclassification of the risk factor with age. Secondly, since BCG was introduced into the LEP population only in 1974, older vaccinated individuals must have received the vaccine when they were already adult and thus already exposed to (infected with) many mycobacteria, perhaps including M. leprae . This observation that BCG vaccination is effective in this population at any age is consistent with the results of the BCG vaccine trial in Papua New Guinea (1), and has important implications for the utility of BCG in leprosy control.
Only 29 (6%) of incident cases were MB. This low proportion is consistent with other reports from Africa (l7) and contrasts with higher proportions of MB cases reported from Asian and Caucasian populations (3). The low percentage may be viewed in the context of current opinions of the role of MB cases as the major sources of M. leprae transmission in the community. Although the low percentage of MB incidence cases contrasts with the high overall incidence rate of disease, it is possible that the intensive case-finding activities during the initial survey identified some PB cases early which would have become MB if they had not been treated at an early stage. Of the 29 MB cases, 16 (55%) were males. This contrasts with the female excess among PB cases, and among all cases combined, and is consistent with the male excess in MB disease reported consistently from other populations. Also consistent with other populations is the observation that MB disease appeared at older ages than PB disease. This sometimes has been ascribed to a longer incubation period for de novo MB disease. Alternatively, it could reflect that MB disease arises as a result of deterioration ("downgrading") and continued bacterial multiplication in PB cases which fail cither to self-cure or to be identified and cured by treatment. The four cases whose MB classification was attributed only to the nerve biopsy may be examples of such a process.
The impact of BCG vaccination on leprosy risk in this population has been discussed elsewhere (15), and we emphasize here only its implications for diagnostic certainty and for overall leprosy incidence. Estimates of vaccine efficacy are highest when restricted to the narrowest (N) diagnostic category (Table 3), reflecting the highest diagnostic specificity in this group. (The alternative explanation, that the probable cases reflect another form of leprosy against which the vaccine is less effective, strikes us as improbable.) On the basis of this observation subsequent analysis of risk factors in the population, and ultimately of the efficacy of KPT vaccines, will concentrate upon the N group cases.
The estimated vaccine efficacy based upon the 62 cases with lesions at first examination was approximately half that in cases with no early lesions at the start (Table 3). Although, given the small numbers, we cannot exclude chance as the explanation for this finding, it may indicate that vaccination is most effective when given earliest, either prior to infection or else early in the infection process. Beyond such hypotheses, the finding carries the important implication that vaccine trials need to record carefully all lesions in trial participants at time of vaccination, and should remove such cases from analyses prior to breaking the vaccine code, in order to provide proper estimates of prophylactic efficacy.
Restriction of these analyses to N (certain) cases with no lesions at the start of follow up provides an estimated vaccine efficacy for BCG of 65%.
The particularly high observed efficacy of BCG against MB disease has implications for pathogenesis as well as control of the disease. It indicates that the "defect" in the immunity of individuals with MB disease can be corrected, and is consistent with the view that MB disease arises by a gradual downgrading of initially PB pathology. It may be that BCG acts by inducing some general enhancement of immunity against M. leprae infection, thereby protecting some potentially PB "cases" entirely, and also shifting some potentially MB cases into the PB category.
If the same incidence rates observed among scar negatives had been obtained in the entire population, we would have expected 596 cases to have arisen during this study period (Fig. 2). The routine BCG program thus appears to have reduced the incidence by (596 - 489)/596 = 18%. This reduction is thus far restricted largely to the younger segment of the population (Tables 1 and 2) since relatively few older adults have ever received BCG vaccination. However, given the evidence for effectiveness for BCG among even the oldest age groups in this population, we expect the impact of BCG in reducing leprosy incidence to increase with progressive aging of the vaccinated cohorts. This trend could be diminished if the effect of BCG in individuals were to wane with time. In this context, the implications of repeating a BCG vaccination, as is currently being evaluated in the Karonga Prevention Trial, are of particular importance (5).
Acknowledgment. The Lepra Evaluation Project was funded primarily by LEPRA (British Leprosy Relief Association) with important contributions from the International Federation of Anti-Leprosy Organizations (ILEP) and the IMMLEP and THELEP components of the UN Development Program/World Bank/ WHO Special Program for Research and Training in Tropical Diseases.
Our special thanks to Chief Kyungu, Dr. Gjalt Boerrigterand Mr. W. Twca, without whose efforts it would not have been possible to start the LEPRA Evaluation Project, and to Drs. A. C. McDougall and S. B. Lucas for providing histopathological support.
REFERENCES
1. BAGSHAWE, A., SCOTT, G. C, RUSSELL, D. A., WIGLEY, S. C, MERIANOS, A. and BERRY, G. BCG vaccination in leprosy: final results of the trial in Karimui, Papua New Guinea. Bull. WHO 67(1989)389-399.
2. DOULL, S. A., GUINTO, R. S., RODRIGUEZ, J. N. and BANCROFT, H. The incidence of leprosy in Cordova and Talisay, Cebu, Philippines. Int. J. Lepr. 10(1942)107-131.
3. FINE, P. E. M. Leprosy - the epidemiology of a slow bacterium. Epidemiol. Rev. 4(1982)161-188.
4. FINE, P. E. M., JOB, C. K., MCDOUGALL, A. C, MEYERS, W. M. and PONNIGHAUS, J . M. Comparability between histopathologists in the diagnosis and classification of leprosy. Int. J. Lepr. 54(1986)614-625.
5. FINE, P. E. M. and PONNIGHAUS, J. M. Background, design and prospects of the Karonga Prevention Trial, a leprosy vaccine trial in northern Malawi. Trans. R. Soc. Trop. Med. Hyg. 82(1988)810-817.
6. FINE, P. E. M., PONNIGHAUS, J. M. and MAINE, N. The distribution and implications of BCG scars, with particular reference to a population in Northern Malawi. Bull. WHO 67(1989)35-42.
7. FINE, P. E. M. and RODRIGUES, L. C. Modern vaccines: mycobacterial diseases. Lancet 335(1990)1016-1020.
8. IRGENS, L. M. Leprosy in Norway-an epidemiological study based on a national patient registry. Lepr. Rev. 51 Suppl. 1(1980)1-130.
9. JESUDASAN, K. , BRADLEY, D. , SMITH, P. G. and CHRISTIAN, M. The effect of intervals between surveys on the estimation of incidence rates of leprosy. Lepr. Rev. 55(1984)353-359.
10. MARTINEZ DOMINGUEZ, V. , GALLEGO GARBAJOSA, P., GYI, M. M., TAMONDONG, C. T., SUNDARESAN, T., BECHELLI, L. M. , LWIN, K. , SANSARRICQ, H. , WALTER, J . and Noussrrou, T. M . Epidemiological information on leprosy in the Singu area of upper Burma. Bull. WHO 58(1980)81-89.
11. MCDOUGALL, A. C, PONNIGHAUS, J. M. and FINE, P. E. M. The histopathological examination of skin biopsies from an epidemiological study of leprosy in northern Malawi. Int. J. Lepr.55(1987)88-98.
12. PONNIGHAUS, J. M. , FINE, P. E. M. and BLISS, L. Certainty levels in the diagnosis of leprosy. Int. J. Lepr. 55(1987)454-462.
13. PONNIGHAUS, J. M. , FINE, P. E. M. , BLISS, L., SLINEY, I. J., BRADLEY, D. J. and REES, R. J. W. The Lepra Evaluation Project (LEP), an epidemiological study of leprosy in northern Malawi. I. Methods. Lepr. Rev. 58(1987)359-375.
14. PONNIGHAUS, J. M., FINE, P. E. M., MAINE, N., BLISS, L., KALAMBO, M. and PONNIGHAUS, I. The Lepra Evaluation Project (LEP) an epidemiological study of leprosy in northern Malawi. II. Prevalence rates. Lepr. Rev. 59(1988)97-112.
15. PONNIGHAUS, J. M., FINE, P. E. M., STERNE, J. A. C, WILSON, R. J., MSOSA, E., GRUER, P. J. K., JENKINS, P. A., LUCAS, S. B., LIOMBA, N. G. and BLISS, L. Efficacy of BCG against leprosy and tuberculosis in northern Malawi. Lancet 339(1992)636-639.
16. RAO, P. S. S., KARAT, A. B. A., KALIAPERUMAL, V. G. and KARAT, S. Incidence of leprosy in Gudiyatham Taluk, South India. Indian J. Med. Res. 60(1972)97-105.
17. STANLEY, S. J. , HOWLAND, C, STONE, M. M. and SUTHERLAND, I. BCG vaccination of children against leprosy in Uganda: final results. J. Hyg. (Camb.) 87(1981)235-248.
18. VANDERVEKEN, M., LECHAT, M. F. , MISSION, C. B. and ANTHONY, V. V. Age-sex-type-specific incidence rates in leprosy. Observations on 45,000 leprosy patients detected in Polambukkam, South India, over a 27-year period. XII International leprosy Congress Proceedings, New Delhi, February 20-25, 1984. New Delhi: International Leprosy Association, n.d., 578-580.
1. Dr.Med., D.T.P.H.; LEPRA Evaluation Project, P.O. Box46, Chilumba, Karonga District, Malawi, Central Africa.
2. LEPRA Evaluation Project, P.O. Box46, Chilumba, Karonga District, Malawi, Central Africa.
3. LEPRA Evaluation Project, P.O. Box46, Chilumba, Karonga District, Malawi, Central Africa.
4. V.M.D.; Communicable Disease Epidemiology Unit, Department of Epidemiology and Population Sciences, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, U.K.
5. Ph.D.; Communicable Disease Epidemiology Unit, Department of Epidemiology and Population Sciences, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, U.K.
6. B.Sc, Communicable Disease Epidemiology Unit, Department of Epidemiology and Population Sciences, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, U.K.
7. B.Sc, Communicable Disease Epidemiology Unit, Department of Epidemiology and Population Sciences, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, U.K.
Reprint requests to Dr. Fine. Current address for Dr. Pönnighaus: Universitats-Hautklinik, 66421 Homburg, Saar, Germany.
Received for publication on 3 August 1993.
Accepted for publication on 26 October 1993.