- Volume 62 , Number 1
- Page: 24–31
Severe pan-sensory neuropathy in leprosy
ABSTRACT
The sensory loss which occurs in leprosy is essentially cutaneous, resulting f rom centripetally ascending infection, the host cellular response and fibrosis, f rom dermal to certain mixed nerves. The hallmark is pain/ temperature and touch/pressure loss. Muscle denervation is a byproduct of mixed nerve involvement. Leprous sensory and motor neuropathy presents a stereotyped picture, with preservation of position sense, noninvolvemcnt of the large girdle muscles, and retained deep tendon reflexes. We report clinical and investigative details of 7 patients (3 males, 4 females) with mild-tomoderate polyneuritic leprosy who manifested severe proprioceptive loss in the upper limbs; the lower limbs were similarly affected in 4 of them. Tendon reflexes were absent in the ataxic limbs. No other cause was found for the ataxia. Electrophysiological studies confirmed damage to large cutaneous and muscle afferents, and a normal EMG pattern in hip and shoulder muscles. Of great interest was the histology of a lumbar sensory ganglion biopsied in a severely disabled patient. There was extensive neuron loss and degeneration and reactive proliferation of capsular cells ("nodules of Nageotte"), an inflammatory focus of lymphocytes, and no bacilli. This suggests to us that the proprioceptive loss in these patients could well be the result of an unusual "leprous ganglionitis." Further clarification of the mechanism ofganglion degeneration and the frequency of inflammation could come f rom immunohistology of tissues f rom African green monkeys with experimental polyneuritic leprosy.RÉSUMÉ
La perte sensorielle qui apparaît dans la lèpre est essentiellement cutanée, résultant d'une infection ascendante centripète, de la réponse cellulaire de l'hôte, et de la libróse, des nerfs du derme à certains nerfs mixtes. La signature en est une perte de sensibilité à la douleur/température et au toucher/pression. La dénervation musculaire est une conséquence annexe de l'atteinte nerveuse mixte. La neuropathie sensorielle et motrice de la lèpre présente une image stéréotypée, avec préservation du sens de la position, la non atteinte des gros faisceaux musculaires, et la conservation des réllexes tendineux profonds. Nous rapportons les détails cliniques et d'investigations de 7 patients (3 hommes, 4 femmes) présentant une lèpre polyneuritique modérée à légère qui ont manifesté une perte proprioceptive légère dans les membres supérieurs; les membres infëreiures étaient affectés de manière similaire chez 4 d'entre eux. Les réllexes tendineux étaient absents dans les membres ataxiques. Aucune autre cause n'a été trouvée pour l'ataxie. Les examens électrophysiologiques ont confirmé des lésions aux gros afférents cutanés et musculaires, et un EMG normal dans les muscles de la hanche et de l'épaule. L'histologie d'un ganglion sensoriel lombaire biopsie chez un patient sévèrement handicapé était d'un grand intérêt. Il y avait une perte et une dégénérescence neuronique étendue et une prolifération réactionnelle de cellulescapsulaires ("nodules de Nageotte"), un foyer inflammatoire à lymphocytes, et pas de bacilles. Ceci nous suggère que la perle proprioceptive chez ces patients pourrait bien être le résultat d'une "ganglionnite léppreuse." Une meilleure compréhension du mécanisme de dégénérescence ganglionnaire et de la fréquence de l'inflammation pourrait venir de l'immunohistologie de tissus provenant de singes verts d'Afrique présentant une lèpre polyneuritique expérimentale.RESUMEN
La pérdida sensorial que ocurre en la lepra es esencialmente cutanea y resulta de la infección centrípeda ascendente, de la respuesta celular del huésped, y de la fibrosis que comienza en los nervios dérmicos y termina por afectar a ciertos nervios mixtos..La característica distintiva del daño es la pérdida de dolor/ temperatura y de tacto/presión. La denervation muscular es una consecuencia de la afección de los nervios mixtos. La neuropatía sensorial y motora de la lepra presenta un cuadro estereotipado, con preservación del sentido de posición, sin afección de los grandes músculos lumbares y con retención de los reflejos de los tendones profundos. Aquí reportamos los detalles clínicos e investigativos de 7 pacientes (3 hombres, 4 mujeres) con lepra polincurítica quienes desarrollaron severa pérdida propiorecepliva en los miembros superiores; los miembros inferiores fueron similarmente afectados en 4 de ellos. Los reflejos tendinosos estuvieron ausentes en los miembros atáxicos. No se encontró ninguna otra causa de la ataxia. Los estudios electrolisiológicos confirmaron el daño de los aferentes cutáneos y musculares, y un patrón electromiográlico normal en los músculos de cadera y hombros. La histología de la biopsia de un ganglion sensorial lumbar de un paciente con afección severa fue de gran interés porque se observó una extensa pérdida neurona, degeneración y proliferación reactiva de las células capsulares ("nodulos de Nageotte"), un foco inflamatorio de linfocitos, y ausencia de bacilos. Esto sugirió que la pérdida propioreceptiva en estos pacientes bien pudo ser el resultado de una rara "ganglionitis leprosa." El estudio ¡nmunohistológico de los tejidos de los monos verdes africanos con lepra polincurítica experimental podría aclarar el mecanismo de la degeneración gan- glionar y la frecuencia de inflamación.Leprosy neuropathy starts in the dermal nerve endings and later involves, in order, dermal, cutaneous sensory and certain mixed nerves by a process of centripetal spread of bacilli and the host cellular response. The preference of the bacilli for cooler areas of the skin and nerves leads to a stereotyped sensory and motor loss which is of diagnostic utility. For example, it is unknown for sensations other than cutaneous (pain/thermal and touch/pressure) and for muscles other than those of the hands, feet, forearm (some) and legs (some) to be involved. The large girdle muscles are not dencrvated, and the tendon reflexes mediated by the affcrcnts from these muscles are well preserved. This "immunity" of certain muscles is presumably the basis of statements that "the typical fully developed pattern of paralysis does not interrupt the ares of the usually elicited stretch reflexes" (13) but Sebille's study of the soleus Hoffmann ("H") reflex suggests that this pathway might sometimes be compromised in leprosy. Other authors are of the view that "Except in the advanced stages of nerve damage . . . the deep reflexes are retained." (1) On the sensory side, Sabin and Jacobson (13) claim that in leprosy "position and vibration are entirely normal except in the unusual or very advanced case in which multiple major nerves in an extremity are affected" (13), while other texts on leprous neuropathy do not even mention sensations such as proprioception and vibration.
We report clinical and other features of seven patients with leprosy who had nerve damage of only a mild or moderate degree but who developed a severe sensory ataxia in addition to the usual cutaneous anesthesia. Also documented is a possible mechanism for this complication not hitherto reported in the literature.
CASE REPORTS
The patients (four females and three males) ranged in age from 26 to 67 years. All patients had polyneuritic (two had no visible skin lesions) leprosy, which we define as neuropathic involvement of all four limbs by the leprous process. None had pure tuberculoid or pure lepromatous leprosy and thus were in the borderline (BT or BL) groups. In all of these patients appropriate investigations were done to exclude other causes of ataxia, e.g., syphilis, vitamin B12 deficiency, diabetes mellitus (only Patient 3 has mild well-controlled diabetes), cervical cord compression, etc.; none had carcinoma. Relevant clinical details are presented and the results of histology and electrophysiology, etc., are shown in The Table.
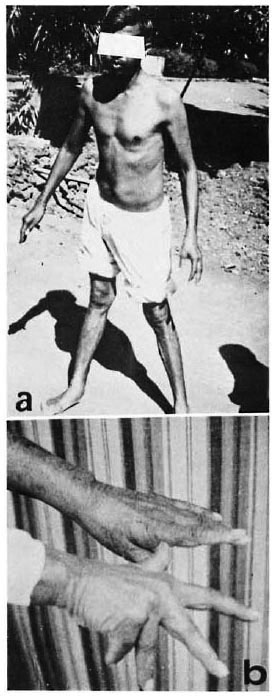
Fig. 1. Clinical evidence of severe proprioceptive loss: a = Patient 2, unable to stand unaided except on a wide base; b = Patient 5, pseudo-athetosis of outstretched hands.
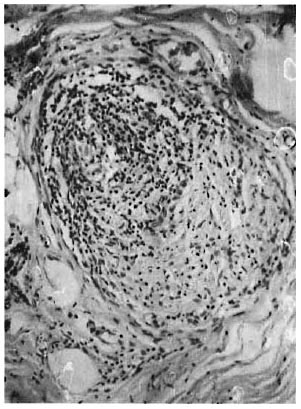
Fig. 2. Patient I, biopsied radial nerve showing BT type of inflammatory exudate (H&E x 200).
Patient 1. A 35-year-old, male, Omani national underwent surgery for hemorrhoids 8 months prior to examination. The history was that a few weeks postsurgery he developed mild ascending parasthesiae, numbness, impaired gait and limb weakness which subacutcly increased in severity in the succeeding months. He also developed neuropathic ulcers in the feet. Examination showed bilateral facial palsy, weak ankle dorsiflexors and good power in the remaining limb muscles; incoordination in all limbs; loss of pain and touch more in the lower than the upper limbs. Vibration and position sense were lost distal to the elbow and impaired to the shoulder; they were absent in the leg. In brief, all sensory modalities were impaired over large areas of the skin, with relative sparing over the back and chest. He had trachoma and corneal opacities. The tendon and plantar reflexes were absent. The peripheral nerves were enlarged. No skin lesions were noted.
The final diagnosis after consideration of the results of investigation (The Table) was "tuberculoid leprosy involving sensory nerves up to the roots."
Patient 2. A 37-year-old male (Fig. 1a) presented with a 6-month history of dull aching in the thighs (left > right); progressive difficulty in walking and numbness in the limbs with "cotton-wool" sensation in the soles, and weakness in dorsiflexing the left foot. Leprosy was detected at another hospital following detection of hypopigmented skin lesions and a radial nerve biopsy. Multidrug therapy (MDT) was started, but the unsteadiness in the hands and feet increased. On examination there was marked loss of pain/temperature and proprioception in the limbs; there were multiple, bilaterally symmetrical, dry, ill-defined, hypopigmented anesthetic macules on the trunk and buttocks; scars of plantar ulcers; weakness (grade 1/2) in ankle dorsiflexors bilaterally; other muscles grade 4; tendon and plantar reflexes absent; and peripheral nerves were moderately enlarged.
Patient 3. A 63-year-old female had developed erythematous lesions on the face and numbness in the feet 6 years prior to examination. A skin smear done in a leprosy hospital was positive for acid-fast bacilli (AFB); her leprosy was classified as BL and she was put on MDT; she felt well, except for recurrent plantar ulceration. Three years later she complained of increasing numbness and clumsiness of the hands with a tendency to drop things, an inability to hold her comb, etc. Examination showed pseudo-athetosis and sensory incoordination of the hands, especially the left hand; there was loss to thermal sensation over the upper limbs and feet; no incoordination was present in the feet; ankle jerks and upper limb reflexes were absent (Fig. 3).
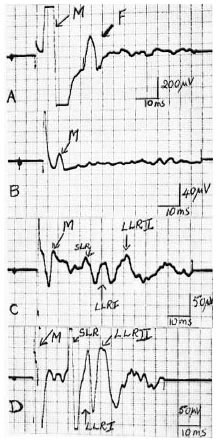
Fig. 3. Patient 3, electrophysiology. Responses in thenar muscle evoked by stimulation of the median nerve at the wrist: A = normal F wave (antidromic activation of anterior horn cells by motor fibers), M response is directly evoked response. 13 = absent H reflex and transcortical reflex (LLR I and II) due to degeneration of large myelinated cutaneous and muscle afferents. C = contrast this with the retained (though delayed) H reflex and transcortical reflex in partially denervated thenar muscle of control patient with the "usual" type of BT leprous neuropathy. D = uninvolved opposite thenar muscle of same control patient showing H and transcortical reflexes at normal latencies.
Patient 4. A 43-year-old female had a 1-year history of development of large and medium-sized hypopigmented anesthetic macules on the face, back of the neck and limbs; chemotherapy for leprosy was given elsewhere; over the following months she complained of increasing numbness in the hands/forearms and inability to button, grasp, etc. The legs were also numb but not unsteady. On examination there was severe cutaneous sensory loss over the limbs, scars of cooking burns on the fingers, and constant pseudo-athetoid movements of the fingers; the finger-nose test showed incoordination; deep tendon reflexes were absent in the upper limbs.
Patient 5. A 67-year-old female had a 3-year history of tingling and frequent anesthetic burns on the fingers; she was diagnosed as having leprosy in her hometown and chemotherapy had been prescribed; the sensory loss increased over the succeeding years, and she became unsteady while walking, especially at night. She also complained of repeated colicky pain in the right hypochondrium. She had marked pseudo-athetosis of the fingers (Fig. lb), incoordination on the finger-nose and hecl-knec tests, and a Romberg's sign on standing with her eyes shut; there were scars of burns on the fingers; motor power was good; tendon reflexes were absent; there were no skin lesions; the cutaneous nerves were moderately enlarged. She was anemic. The etiology of the pain over the area of the right hypochondrium was not discovered, but the patient reported relief after alcohol injection in the overlying skin.
Patient 6. A 34-year-old male presented at the leprosy clinic with several well-defined, hypopigmented, anesthetic macules. Some cutaneous nerves were enlarged. Skin smears were negative for AFB; the skin lesions responded well to chemotherapy. About 6 months later he returned with progressive clumsiness in buttoning, tying, etc. The lower limbs were asymptomatic. He displayed moderate sensory-type incoordination on the finger-nose test; could not button his shirt without visual input; the hand muscles were well preserved; there was a burn scar on the right index finger.
Patient 7. A 26-year-old female had a 3-year history of progressive numbness in the hands and fect, pain radiating from the right lumbo-sacral region to the foot, difficulty in buttoning and easy loss of balance. She was not known to have leprosy. On examination she had a generalized shininess of the skin; there were no macules; she had plantar ulcers; her peripheral nerves showed mild-to-modcrate enlargement; motor power by and large was normal; there was anesthesia to pin-prick, touch, and temperature in a glove-and-stocking distribution; there was severe sensory ataxia in the upper and lower limbs, and the reflexes were absent; the face and pupils were normal (Fig. 4).
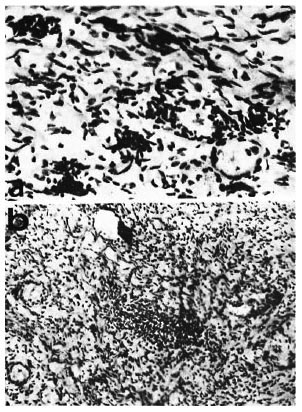
Fig. 4. Patient 7, lumbar ganglion biopsy (photomicrographs supplied by Dr. D. K. Dastur); AFB were not detected: a = capsular cell proliferation ("nodules of Nageotte") and degeneration of large and small neurons (Fite-Faraco stain x 250). b = inflammatory focus of lymphocytes in the midst of degenerating neurons suggesting "ganglionitis" (Fite-Faraco stain x 75).
DISCUSSION
Clinical. The rarity of the syndrome described here is suggested by the fact that a) the seven patients were seen over a period of 20 years, and b) the peculiar neurologic defect led all but three patients to be referred initially to nonleprosy specialists (neurologists, etc.) who permitted us to study these patients after due consideration and investigation for other neurological diseases.
All of these patients had clinically apparent or biopsy-confirmed leprosy neuropathy, with or without bacilli. All were in the borderline leprosy category and, thus, were immunologically capable of mounting a cellmediated response to leprosy antigen.
The rarity of an ataxic syndrome in leprosy warranted exclusion of other neurologic conditions. Commonly seen diseases, such as vitamin B12 deficiency, syphilis, cord compressions, and malignancies, were excluded by appropriate investigations. In Patient 3 with mild diabetes mellitus, "diabetic pseudo-tabes" was considered unlikely since the more vulnerable lower limbs were not ataxic.
Less common causes of large-fiber sensory loss (e.g., sarcoid neuropathy, Sjogren's syndrome, neurotoxins) were excluded on historical and clinico-histological grounds since none of these diseases simultaneously exhibits nerve enlargement (with or without skin patches), co-existent small-fiber sensory neuropathy (all our patients had this), and granulomatous endoncurial/perineurial inflammation.
In Patient 7 (from whom the ganglion biopsy was obtained) a subacute sensory neuronopathy ("nonmalignant inflammatory sensory polyganglionopathy"), superimposed on her BL leprosy neuropathy, was a possibility but, in our view, not a probability. Her history pointed to a simultaneous onset and progression of large- and small-fiber sensory loss, and it would be stretching credulity to believe that two entirely distinct, predominantly sensory, neuro(no)pathies developed in the same timeframe. A similar coincidence of cutaneous and proprioceptive loss was reported by Patients 1, 2 and 5.
Chemotherapy, or the absence of it, had no bearing on the development of the sensory ataxia. In the patients in whom followup examination was possible, no change was detected in the ataxia, although some had learned to compensate for the loss of position sense with increased visual input.
To us the evidence seems strong that severe proprioceptive loss in leprosy is manifested when the neuropathy is not "advanced." We consider that good motor power is necessary for the manifestation of pseudo-athctosis and kinesthetic loss, contrary to the statements of Sabin and Swift (13).
Electrophysiology. Electrophysiology also documented the inability of the large sensory afferents (from cutaneous, muscle and tendon sources) to mediate reflexes and somatosensory responses. The motor fibers in the hand muscles for example, could conduct both orthodromically and antidromically.
Somatosensory evoked responses could not be recorded on median nerve stimulation either at the wrist or the elbow.
The large muscles of the hip and shoulder whenever studied showed no denervation.
The clinical and electrophysiologic evidence was consistent with the view that the proprioceptive loss resulted from pathology at a proximal site in the sensory pathway, i.e., the spinal ganglion or sensory roots.
Histology. Muscle spindle denervation has been demonstrated in leprosy by the spread of inflammation from motor and intramuscular nerves to the fusal nerves and the spindle capsule (8, 9). AFB have been seen in human (9) and in leprous mouse muscle spindles (5, 6), but probably are not the destructive factor per se .
In our ataxic patients the occurrence of a pathogenetic severe and preferential "spindle neuritis" is highly unlikely since neighboring motor fibers were relatively unscathed (motor power, as stressed above, was good).
The lumbar dorsal root ganglion biopsy in the severely disabled Patient 7 showed clear neuronal death and degeneration, proliferation of the satellite cells ("residual nodules of Nageotte") and, most significantly, a lymphocytic inflammatory area although no AFB. Some neurons appeared intact, but their functional status was questionable. For the reasons mentioned above, we regard this as "leprous ganglionitis," a feature not reported before.
The microscopic picture of the dorsal ganglia in leprosy was ably discussed and reviewed by Lumsden (7). Bacillation of the spinal ganglia (more correctly, the satellite capsular cells which are congeners of Schwann cells, themselves favored for bacillary infestation) was described by the pioneer pathologists in the "golden age of histological research in leprosy." Lumsden emphasized that, as in the lepromatous nerve, bacilli in the investing capsular cells may not compromise neuronal function. (Inflammation and fibrosis are more potent than AFB in nerve destruction.) Khanolkar (6) depicted various stages of neuronal degeneration in autopsicd ganglia, in addition to AFB in the capsular cells. He was of the opinion that the histological picture resulted from "retrograde" neuronal degeneration. Inflammation was not mentioned.
If leprous "ganglionitis" is indeed the mechanism for the kinesthetic sensory deficit in our patients, some questions come to mind. Why is it so rare in cellular immunecompetent leprosy when there is no natural ganglion-blood barrier to breach? How does the antigen enter the ganglia of muscles not dencrvatcd in this disease, i.e., quadriceps, biceps, etc., (which were arcflexic)? If the route is hematogenous, why are ganglia of the upper limb at greater risk than those of the lower? (lower body temperature?)
The interactions between Mycobacterium leprae , the immune system, and the sensory ganglia could perhaps be clarified by application of newer techniques such as immuno-histology to tissue from leprosy-infected primates (particularly African green monkeys which are predisposed to developing polyneuritic leprosy), and to ganglia from all spinal levels in human post mortem material.
Acknowledgment. We thank Drs. B. S. Singhal and N. H. Wadia (neurologists). Dr. P. K. Jhawar (surgeon), and Dr. H. O. Bulchand (leprosy physician) for permission to examine and study their patients who exhibited this syndrome. We also thank Dr. D. K. Dastur (neuropathologist) for a reappraisal of the nerve and ganglion pathology of Patient 7, and for permission to reproduce his report.
REFERENCES
1. BROWNE, S. G. Leprosy-clinical aspects of nerve involvement. In: Topics on Tropical Neurology . Hornabrook, R. W., ed. Philadelphia: F. A. Davis Company, 1975, pp. 1-12.
2. DASTUR, D . K . The nervous system in leprosy. In: Scientific Approaches to Clinical Neurology . Goldensohn, E. and Appel, S., cds. New York: Lea & Febiger, 1977, pp. 1456-1493.
3. DASTUR, D . K . Leprosy (an infectious and immunological disorder of the nervous system). Handbook Clin. Neurol. 33(1978)421-468.
4. DEUSCHL, G., SCHENK, E . and LUCKING, C. H. Long latency responses in human thenar muscles mediated by fast-conducting muscle and cutaneous aflercnts. Neurosci. Lett. 55(1985)361-366.
5. EDWARDS, R. P. An ultrastructural study of neuromuscular spindles in normal mice: with reference to mice and man infected with Mycobacterium leprae . J. Anat. 120(1975)149-168.
6. KHANOLKAR, V. R. Perspectives in pathology of leprosy. Indian J. Med. Sci. 9 Suppl. 1(1955)1-52.
7. LUMSDEN, C. E. Leprosy and the Schwann cell in vivo and in vitro . In: Leprosy in Theory and Practice . Cochrane, R. G. and Davcy, T. F., eds. Bristol: John Wright and Son, 1964, pp. 221-250.
8. PANDYA, S. S. and CHULAWALA, R. G. Innervation of muscle in leprosy with particular reference to the muscle spindle. Int. J. Lepr. 43(1975)32-35.
9. PATEL, A. N., LALITHA, V. S. and DASTUR, D. K. The spindle in normal and pathological muscle; an assessment of the histological changes. Brain 91(1968)737-750.
10. PETTIT, J. H. S. Neurology of leprosy. In: Tropical Neurology . Spillane, J. D., ed. Oxford: Oxford University Press, 1973, pp. 321-344.
11. SABIN, T. D., SWIFT, T. H. and JACOBSON, R. R. Leprosy. In: Peripheral Neuropathy . 3rd edn. Dyck, P. J., Thomas, P. K. , Griffin, J., Low, P., and Poduslo, J. P., cds. Philadelphia: W. B. Saunders Company, 1992, pp. 1354-1379.
12. SEBILLE, A. The Hoffmann reflex of the solcus muscle: a study in leprosy. J. Neurol. Sci. 45(1980)373-378.
13. WOLF, R. H., GORMUS, B. J., MARTIN, L. N., WALSH, G. P., MEYERS, W. M. and BINFORD, C. H. Experimental leprosy in three species of monkeys. Science 227(1985)529-531.
1. M.B.B.S., Acworth Leprosy Hospital Society for Research, Rehabilitation and Education in Leprosy, Bombay 400031, India, and EMG Department, Bombay Hospital, Bombay 400020, India. W.
2. M.B.B.S., Acworth Leprosy Hospital, Bombay 400031, India.
Reprint requests to Dr. Pandya, RRE Society, Acworth Leprosy Hospital, Bombay 400031, India.
Received for publication on 19 August 1993.
Accepted for publication in revised form on 10 November 1993.
