- Volume 62 , Number 1
- Page: 37–42
Further study of the effectiveness of single doses of clarithromycin and minocycline against Mycobacterium leprae in mice
ABSTRACT
The anti- Mycobacterium leprae activities of single doses of rifampin (RMP), clarithromycin (CLARI), or minocycline (MINO) alone, and various combinations of CLARI + MINO were determined in immunocompetent mice by the kinetic method. A single dose of RMP 10 mg/kg, CLARI 100 mg/kg or 200 mg/kg, MINO 25 mg/kg or 50 mg/ kg alone, or various combinations of CLARI + MINO were active. RMP was more active than the other treatments; the activity of CLARI 100 mg/kg was greater than that of 50 mg/kg, but did not differ significantly f rom that of 200 mg/kg; MINO 50 mg/kg was more active than 25 mg/kg; and none of the combinations of CLARI + MINO was more active than any of the stronger components administered alone. Therefore, both CLARI and MINO may be applied, either alone or in combination, as components of monthly administered, fully supervised, multidrug regimens for the treatment of multibacillary leprosy. Taking into account the effectiveness of the drugs and the comparative pharmacokinetic data, we propose that the optimal dosage in human trials is CLARI 1000 mg per month or MINO 200 mg per month.RÉSUMÉ
L'activité anti- Mycobacterium leprae de doses unique de rifampicine (RMP), clarithromycine (CLARI), ou minocycline (MINO) administrées isolément, et diverses combinaisons de CLARI + MINO a été déterminée par la méthode cinétique chez des souris immunocompétentes. Une dose unique de RMP 10 mg/ kg, de CLARI 100 mg/kg ou 200 mg/kg, de MINO 25 mg/kg ou 50 mg/kg administrées seules, ou diverses combinaisons de CLARI + MINO étaient actives. La RMP était plus active que les autres traitements; l'activité de CLARI 100 mg/kg était plus grande que celle de 50 mg/kg, mais ne différait pas significativement de celle de 200 mg/kg; la MINO à 50 mg/kg était plus active que celle à 25 mg/kg; et aucune des combinaisons de CLARI + MINO n'était plus active qu'aucun des composants les plus puissants administrés seuls. Dés lors, la CLARI et la MINO peuvent être utilisées, seules ou en combinaison, comme composants de régimes polychimiothérapeutiques entièrement supervisés administrés mensuellement pour le traitement de la lèpre multibacillaire. Prenant en compte l'efficacité des médicaments et les données comparatives de pharmacocinétique, nous proposons comme dosage optimal pour les essais chez l'homme 1000 mg par mois de CLARI ou 200 mg par mois de MINO.RESUMEN
Utilizando cl método cinético y ratones inmunocompetentes, se determinó la actividad anti- Mycobacterium leprae de dosis únicas de rifampina (RMP), de claritromicina (CLARI), o de minociclina (MINO), administradas solas o en varias combinaciones de CLARI + MINO. Una sola dosis de RMP ( 10 mg/kg), de CLARI (100 ó 200 mg/kg), o de MINO (25 ó 50 mg/kg), y varias combinaciones de CLARI + MINO fueron activas contra cl M. leprae pero la RMP fue la más activa. La actividad de CLARI fue mayor a la dosis de 100 mg/kg que a la de 50 mg/kg pero no difirió significativamente de aquella de 200 mg/kg; MINO a 50 mg/ kg fue más activa que a 25 mg/kg. Ninguna de las combinaciones de CLARI + MINO fue más activa que los fármacos más potentes administrados solos. Por lo tanto la CLARI como la MINO podrían emplearse, solas o combinadas, como componentes de la poliquimioterapia administrada mensualmente para el tratamiento de la lepra multibacilar. Tomando en cuenta la efectividad de las drogas y los datos farmacocinéticos comparativos, proponemos que la dosis óptima en los ensayos con humanos podría ser de 100 mg/mes de CLARI o de 200 mg/mes de MINO.Since the introduction of multidrug therapy (MDT) (18), more than 4 million leprosy patients in the world have been or are being treated with the regimens (19). In addition to the monthly supervised administration of rifampin (RMP), which is the backbone of the MDT regimens for both paucibacillary (PB) and multibacillary (MB) leprosy, the regimens also contain daily self-administered component(s): dapsone for PB, and dapsonc plus clofazimine for MB leprosy (19). However, in Karigiri, South India, one of the best leprosy programs in the world, and a place in which clofazimine is well accepted by the patients because of their dark skin, nearly 30% of the MB patients did not take their prescribed dapsone and clofazimine properly (2). This suggests that, simply because of noncompliance, it is still possible to develop RMP resistance in a program in which MDT is implemented. The risk might be reduced significantly if it were possible to develop a fully supervised multidrug regimen such that all components are administered once monthly under supervision together with RMP. The experience in the implementation of MDT in different parts of the world indicates that monthly outpatient contact for supervised administration of drugs is, in general, highly accepted.
The basic requirements for a component which may be given once monthly for the treatment of leprosy are: a) a single dose displays a certain degree of bactericidal activity against Mycobacterium leprae , and b) the dosage is well tolerated (11). Recently, we showed by the proportional bactericidal method (2) that a single dose of clarithromycin (CLARI) (5, 9, 10 ) 100 mg/kg plus minocycline (MINO) (6, 7, 9, 10) 25 mg/kg displayed significant bactericidal activity against M. leprae in mice (11).
In the current experiment, using the kinetic method (15-17) we have determined the activity of the single dose of CLARI + MINO against another strain of M. leprae , analyzed the contributions of the individual drugs, and compared the activities of various dosages of CLARI and MINO alone and in combination.
MATERIALS AND METHODS
M. leprae . M. leprae Strain no. 17547 was isolated from a previously untreated lepromatous patient and maintained in mouse passage. The strain was fully susceptible to both RMP and dapsone. An inoculum containing 5 X 103 M. leprae per 0.03 ml was prepared (14).
Mice. Four-hundred female, 4-week old, immunocompetent Swiss mice were purchased from a local supplier.
Mouse inoculation with M. leprae . The mice were inoculated with 5 X 103 M. leprae in each hindfoot pad. After inoculation, the mice were randomly allocated to 13 groups: an untreated group of 40 mice and 12 treated groups of 30 mice each.
Treatments. The control mice were not treated during the entire period of the experiment. For the treated groups, at day 70 (D70) after inoculation, the various regimens were administered by gavagc only once as shown in The Table. The drugs were suspended in 0.05% agar-distilled water and administered in the following dosages: RMP 10 mg/kg, CLARI 50, 100 and 200 mg/kg, MINO 25 and 50 mg/kg. The dosages of the drugs were selected to provide concentrations in serum or areas under the concentration time curves in mice comparable to those achievable in man with clinically tolerated dosages, i.e., RMP 600 mg (8), CLARI 500, 1000 and 2000 mg (Abbott Laboratories, unpublished data and 3, 4, 10) and MINO 100 (6, 10) and 200 mg daily.
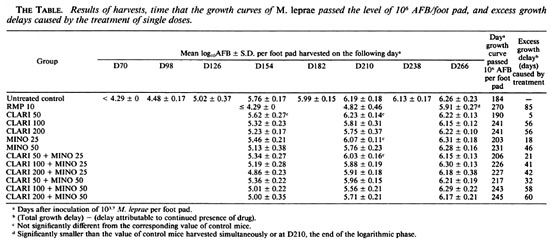
Harvests. Starting from D70 after inoculation, i.e., the same day treatments were begun, the soft tissues of eight inoculated foot pads of the control mice were harvested (14) individually, and the process was repeated with the same number of foot pads at 28-day intervals until D266, when the last harvest from the treated groups were performed (17). The harvests of the treated groups, also eight foot pads per group, were begun on D154, when multiplication of M. leprae was unequivocally demonstrated in all foot pads of the control mice, i.e., harvests yielded > 105 acid-fast bacilli (AFB) per foot pad, and were repeated at 56-day intervals until D266, when the mean number of AFB per foot pad of all treated groups was around 106, i.e., the growth curves of M. leprae had reached the plateau level (15).
Calculations and statistical analysis. The results of the harvests were analyzed by the Student's t test; differences between groups were considered significant at the 95% level of confidence. The growth delay (l6-18) caused by each treatment was calculated graphically by comparing the time the growth curves of M. leprae in treated mice passed 106 AFB per foot pad with that in control mice. The excess growth delay, in days, was calculated by deducting the delay attributable to continued presence of the drug from the total growth delay. Because of the relatively short half-lives of the tested drugs in the mice, these drugs should have been present no more than 1 day after a single administration. Taking into account the statistical significance of the difference of the numbers of AFB between the control and treated groups, the minimal excess growth delay of an effective treatment is 20 days in the current experiment.
RESULTS
As shown in The Figure and The Table, the growth curves of M. leprae in the groups, especially in the control mice, were very smooth with a narrow range of standard deviations, a prerequisite for the measurement of anti- M. leprae activity by the kinetic method. In control mice, the mean number of AFB per foot pad increased progressively from D98 to D210, and passed 106 AFB per foot pad 184 days after inoculation; one may easily identify the lag, logarithmic and plateau phases (14) of multiplication of M. leprae .
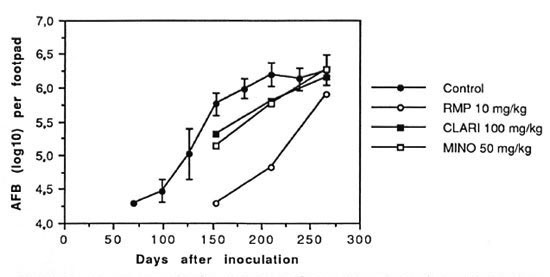
The figure. Growth curves of M. leprae in foot pads of untreated controls and mice treated with a single dose of rifampin (RMP) 10 mg/kg, clarithromycin (CLARI) 100 mg/kg or minocycline (MINO) 50 mg/kg. Drugs were administered by gavage 70 days after inoculation. Each point represents the mean of 8 foot pads; error bars represent standard deviations. The curves in the control group and mice treated with RMP, CLARI or MINO passed 106 AFB per foot pad on day 184, 270, 241 and 231, respectively.
The growth curves in the treated groups lagged behind that of the control group. However, M. leprae resumed multiplication sooner or later in all treated groups, and their growth curves were basically parallel to those of the control mice, another indication of the regularity of multiplication of M. leprae (The Figure, The Table). On D266, except for the groups treated with a single dose of RMP, the mean number of AFB per foot pad of all treated groups was greater than 106, indicating that the growth curves had reached the plateau phase.
The mean numbers of AFB in the mice administered a single dose of RMP were significantly smaller than those in the control mice at all three harvests. The excess growth delay in this group was 85 days, the longest among all treated groups in the experiment.
Among the mice treated with a single dose of CLARI or MINO alone (The Table), CLARI in a dosage of 50 mg/kg was virtually inactive because the numbers of AFB harvested on all three occasions were practically the same as those in control mice, and the excess growth delay was only 5 days. MINO in a dosage of 25 mg/kg displayed a modest degree of activity; the mean number of AFB was significantly smaller than that in control mice only on D154 (p < 0.01) and the excess growth delay, 18 days, was only marginally significant. However, when the single dose of CLARI was increased from 50 mg/kg to 100 mg/kg, or of MINO from 25 mg/kg to 50 mg/kg, the mean numbers of AFB on both D154 and D210 became significantly smaller (p < 0.05 and < 0.01, respectively), and the excess growth delay was substantially prolonged. Both the mean numbers of AFB and the excess growth delays were very similar in the mice administered CLARI in single doses of 100 and 200 mg/kg (p > 0.05). The mean number of AFB in mice treated with MINO 50 mg/ kg was significantly smaller than that in mice administered CLARI 50 mg/kg (p < 0.01), and did not differ significantly from those in mice administered CLARI 100 or 200 mg/kg (p > 0.05), again demonstrating thaton a weight-to-wcight basis MINO is more active than CLARI (l0).
As in the previous experiment (11), bactericidal activity against M. leprae was observed in all six groups treated with single doses of various combinations of CLARI + MINO (The Table). However, except for the D154 harvests from the mice administered the combination of CLARI 200 mg/kg + MINO 25 mg/kg, none of the mean numbers of AFB in mice treated with the combinations was significantly smaller than those observed when the stronger components had been administered alone (p > 0.05).
On D154 and D210, the mean numbers of AFB in mice treated with a single dose of CLARI, MINO, or CLARI + MINO, regardless of the dosages, were significantly greater than those in mice treated with a single dose of RMP 10 mg/kg. In addition, the excess growth delays in these groups were shorter than those in the RMP group, indicating that the activities of single doses of CLARI and MINO, either alone or in combination, were less than that of a single dose of RMP.
DISCUSSION
The current experiment demonstrated by the kinetic method that a single dose of the combination CLARI + MINO was active against M. leprae in the mouse foot pad system, confirming the finding in the earlier experiment (11). In fact, a single dose of cither of the components alone was also active. Since the powerful bactericidal activity against M. leprae in humans and in mice of a single dose of RMP was first demonstrated (10, 13), this is probably the first time that significant anti- M. leprae activity of a single dose of another drug has been demonstrated.
In our previous experiment using the proportional bactericidal method, additive effects were displayed by 20 daily doses of the combination of CLARI (50 mg/kg) and MINO (25 mg/kg) (10). However, neither additive nor antagonistic effects were observed in the current experiment when a single dose of CLARI was combined with a single dose of MINO, regardless of the dosages of the components. Therefore, CLARI and MINO may be administered once monthly, either separately or in combination, in fully supervised regimens, depending upon the objectives of the combined regimens (11).
Tolerance of the treatment is one of the important considerations in designing the composition of a combined regimen. The dosages of drugs should be selected considering the effectiveness of individual components and the tolerance of the combination by the majority of patients. Because the anti- M. leprae activity of a single dose was significantly greater when the dosage of CLARI was increased from 50 to 100 mg/ kg or when that of MINO was increased from 25 to 50 mg/kg, the activity of CLARI 200 mg/kg was virtually the same as that of CLARI 100 mg/kg, and considering the comparative pharmacokinetic data in mice and in humans (Abbott Laboratories, unpublished data and 3, 4, 6, 10), we propose that the optimal dosages for monthly administration to patients be CLARI 1000 mg and MINO 200 mg, both dosages being well tolerated in the treatment of other clinical conditions. Of course, even before clinical trials of monthly administered, fully supervised MDT regimens are organized, pilot studies should be conducted to demonstrate the anti- M. leprae activity of a single dose of CLARI 1000 mg or MINO 200 mg among patients with previously untreated lepromatous leprosy.
The kinetic method cannot distinguish clearly between bacteriopausal and bactericidal activity (18). However, the bactericidal activity of a single dose of the combination CLARI + MINO has been well demonstrated by the proportional bactericidal method (11). In addition, in the current experiment the results of larger dosages of CLARI or MINO alone were very similar to those of CLARI + MINO, and the excess growth delays corresponded to several generation times of M. leprae in the mouse foot pad system (12). Therefore, it is reasonable to assume that a single larger dose of CLARI or MINO alone also displayed bactericidal activity.
Acknowledgment. J.-H . Xiong was partly funded by the Fondation Simone et Cino del Duca, Paris, France.
REFERENCES
1. COLSTON, M. J., HILSON, G. R. F. and BANERJEE, D. K. The "proportional bactericidal test": a method for assessing bactericidal therapy on the growth of Mycobacterium leprae in mice. Lepr. Rev. 49(1978)7-15.
2. ELLARD, G. A., PANNIKAR, V. K., JESUDASAN, K. and CHRISTIAN, M. Clofazimine and dapsone compliance in leprosy. Lepr. Rev. 59(1988)205-223.
3. FERNANDES, P. B., BAILER, R., SWANSON, R., HANSON, C. W., MCDONALD, E., RAMER, N., HARDY, D., SHIPKOWITZ, N., BOWER, R. R. and GADE, E. In vitro and in vivo evaluation of A-56268 (TE-031), a new macrolide. Antimicrob. Agents Chemother. 30(1968)865-873.
4. FERNANDES, P. B., HARDY, D. J., MCDANIEL, D., HANSON, C. W. and SWANSON, R. N. In vitro and in vivo activities of clarithromycin against Mycobacterium avium. Antimicrob. Agents Chemother. 33(1989)1531-1534.
5. FRANZBLAU, S. G. and HASTINGS, R. C. In vitro and in vivo activities of macrolides against Mycobacterium leprae . Antimicrob. Agents Chemother. 32(1988)1758-11762.
6. GELBER, R. H. Activity of minocycline in Mycobacterium /cyww-infected mice. J. Infect. Dis. 156(1987)236-239.
7. GELBER, R. H., FUKUDA, K., BYRD, S., MURRAY, L. P., Siu, P., TSANG, M. and REA, T. H. A clinical trial of minocycline in lepromatous leprosy. Br. Med. J. 304(1992)91-92.
8. GROSSET, J. The sterilizing value of rifimpicin and pyrazinamide in experimental short course chemotherapy. Tubercle 56(1978)287-297.
9. Ji, B., JAMET, P., PERANI, E. G., BOBIN, P. and GROSSET, J.-H. Powerful bactericidal activities of clarithromycin and minocycline against Mycobacterium leprae in lepromatous leprosy. J. Infect. Dis. 168(1993)188-190.
10. Ji, B., PERANI, E. G. and GROSSET, J.-H. Effectiveness of clarithromycin and minocycline alone or in combination against experimental Mycobacterium leprae infection in mice. Antimicrob. Agents Chemother. 35(1991)579-581.
11. Ji, B., PERANI, E. G., PETINON, C. and GROSSET, J.-H. Bactericidal activities of single or multiple doses of various combinations of new antileprosy drugs and/or rifampin against M. leprae in mice. Int. J. Lepr. 60(1992)556-561.
12. LEVY, L. Studies of the mouse foot pad technique for cultivation of Mycobacterium leprae . 3. Doubling time during logarithmic multiplication. Lepr. Rev. 47(1976)103-106.
13. LEVY, L., SHEPARD, C. C. and FASAL, P. The bactericidal effect of rifampin on M. leprae in man: a) single doses of 600, 900 and 1200 mg; and b) daily doses of 300 mg. Int. J. Lepr. 44(1976)183-187.
14. SHEPARD, C. C. The experimental disease that follows the injection of human leprosy bacilli into foot pads of mice. J. Exp. Med. 112(1960)445-454.
15. SHEPARD, C. C. A kinetic method for the study of the activity of drugs against Mycobacterium leprae in mice. Int. J. Lepr. 35(1967)429-435.
16. SHEPARD, C. C. Further experience with the kinetic method for the study of drugs against Mycobacterium leprae in mice; activities of DDS, DFD, ethionamide, caprcomycin and PAM 1392. Int. J. Lepr. 37(1969)389-397.
17. SHEPARD, C. C. Statistical analysis of results obtained by two methods for testing drug activity against Mycobacterium leprae . Int. J. Lepr. 50(1982)96-101.
18. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Scr. 675.
19. WORLD HEALTH ORGANIZATION. Leprosy situation in the world and multidrug therapy coverage. Wkly. Epidemiol. Ree. 67(1992)153-160.
1. M.D.;B.Bactériologie et Virologie, Faculté de Médecine Pitie-Salpctrierc, 91 Boulevard de l'Hopital, 75634 Paris 13, France.
2. M.D.; Bactériologie et Virologie, Faculté de Médecine Pitie-Salpctrierc, 91 Boulevard de l'Hopital, 75634 Paris 13, France.
3. Technical Officer; Bactériologie et Virologie, Faculté de Médecine Pitie-Salpctrierc, 91 Boulevard de l'Hopital, 75634 Paris 13, France.
4. Technical Officer; Bactériologie et Virologie, Faculté de Médecine Pitie-Salpctrierc, 91 Boulevard de l'Hopital, 75634 Paris 13, France.
5. M.D., Bactériologie et Virologie, Faculté de Médecine Pitie-Salpctrierc, 91 Boulevard de l'Hopital, 75634 Paris 13, France.
Reprint request to Dr. Grosset.
Received for publication on 12 July 1993.
Accepted for publication in revised form on 27 October 1993.