- Volume 62 , Number 1
- Page: 43–7
Therapeutic efficacy of benzoxazinorifamycin, KRM-1648, in combination with other antimicrobials against Mycobacterium leprae infection induced in nude mice
ABSTRACT
In this study, the in vitro and in vivo anti- Mycobacterium leprae activity of the newly developed benzoxa/inorifamycin, KRM1648, in combination with clofazimine (CFZ) or dapsonc (DDS) was evaluated. in vitro anti- M. leprae activities of KRM-1648, CFZ, and DDS along with their combinations were measured by the BACTEC 460 TB System. KRM-1648 (0.01 µ g/ml), CFZ (0.5 µ g/ml), and DDS (2.0 µ g/ml) exhibited a significant anti- M. leprae activity, reducing growth index (GI) values by 78%, 30%, and 35% by day 18, respectively. Combinations of KRM-1648 with cither CFZ or DDS, or both caused only a slight increase in the efficacy. BALB/c nude mice infected subcutancously with 1 X 106 of M. leprae Thai-53 strain and test drugs were given to mice by gavage once daily six times per week for up to 50 days, f rom day 31 to day 80. Animals were observed for the growth of organisms in the hindfoot pad during the 12 months following infection. KRM-1648 given at the dose of 0.001 mg/mouse exhibited potent antileprosy activity. KRM-1648 exhibited a significant combined effect with cither CFZ or DDS, or both against M. leprae infection, except that there was no significant difference in efficacy between KRM-1648 + CFZ and CFZ alone. Furthermore, the efficacy was most increased in the three-drug regimen KRM-1648 + CFZ + DDS.RÉSUMÉ
Dans cette étude, on a évalué l'activité anti- Mycobacterium lepraein vitro et in w'vodu nouveau composé qu'est la benzoxazinorifamycine, le KRM-1648, en combinaison avec la clofaziminc (CFZ) ou la dapsone (DDS). Les activités in vitro du KRM-1648, du CFZ et de la DDS, ainsi que de leurs combinaisons ont été mesurées par le système BACTEC 460 TB. Le KRM 1648 (0,01 µ g/ml), le CFZ (0, 5 µ g/ml) et la dapsonc (2,0 µ g/ml) ont montré une activité anti- M. leprae significative, réduisant les valeurs de l'indice de croissance (IC) de respectivement 78%, 30% et 35% au dixhuitième jour. Les combinaisons du KRM-1648 avec le CFZ, la DDS ou les deux n'ont entraîné qu'une légère augmentation de l'efficacité. Des souris nues BALB/c ont été infectées par voie sous-cutanée avec 1 x 106 M. leprae de la souche Thai-53, et les substances testées leur ont été données par gavage une fois par jour et six jours par semaine jusqu'à 50 jours, du jour 31 au jour 80. On a observé pour la croissance des organismes dans le coussinet des pattes postérieures des animaux durant les 12 mois qui ont suivi l'infection. Le KRM1648 à la dose de 0,001 mg souris a montré une puissante activité anti-lépreuse. Le KRM-1648 a montré un effet combiné significatif avec le CFZ, la DDS, ou les deux, vis-à-vis de l'infection à M. leprae , si ce n'est qu'il n'y avait pas de différence significative d'efficacité entre le KRM-1648 + CFZ et le CFZ seul. De plus, l'efficacité était maximale pour le régime comprenant les trois substances KRM-1648 + CFZ + DDS.RESUMEN
En este estudio se evaluó in vivo e in vitro , la actividad anti- Mycobacterium leprae de la droga benzoxazinorifamicina (KRM-1648) administrada sola, o combinada con clofazimina (CFZ) o con dapsona (DDS). La actividad de esta droga y sus combinaciones se midió por el sistema BACTEC 460 TB. Hacia el día 18, la KRM-1648 (0.01 µ g/ml), la CFZ (0.5 µ g/ml) y la DDS (2.0 µ g/ml), exhibieron una significante actividad anti-M. leprae al reducir su índice de crecimiento en un 78%, un 30%, y un 35%, respectivamente. La combinación de KRM-1648 con CFZ, con DDS, o con ambas drogas, condujo sólo a un ligero incremento de su eficacia. Para los estudios in vivo , se inyectaron ratones BALB/c desnudos con 1 x 106 M. leprae de la cepa Thai-53 por la vía subcutánea y 31 días después se trataron con las drogas administradas junto con la dicta, una vez al día, 6 veces por semana, hasta completar 50 días. Los animales se examinaron para establecer la multiplicación de los bacilos en las almohadillas plantares durante los 12 meses siguientes a la infección. La droga KRM-1648 administrada a la dosis de 0.001 mg por ratón exhibió una potente actividad antileprosa. Combinada con CFZ, con DDS, o con ambas, la droga exhibió un significante efecto antilcproso combinado, excepto que no hubo ninguna diferencia significativa entre la eficacia de la mezcla KRM 1648 + CFZ y la eficacia de la CFZ sola. La eficiencia antilcprosa se incrementó en forma máxima cuando se administraron las 3 drogas juntas.Multidrug therapy (MDT) consisting of rifampin (RMP), clofazimine (CFZ), and diaminodiphenylsulfone (DDS, dapsonc) is considered to be the most effective treatment for patients with leprosy (10). However, it takes from at least 6 months to more than 4 years of treatment to achieve appreciable results in the control of multibacillary leprosy, even with this multidrug regimen (3). The development of new protocols which could provide more rapid therapy for leprosy patients and that contain other types of antileprosy drugs is, therefore, considered urgent.
The new benzoxazinorifamycin derivative KRM-1648 (Kaneka Corporation, Hyogo, Japan) has excellent in vitro antimycobacterial activities, and is much more potent than RMP (1, 8). KRM-1648 exhibited a potent therapeutic efficacy against Mycobacterium avium complex infections induced in mice and rabbits (1, 9). Moreover, we previously found that KRM-1648 exhibited much more potent therapeutic efficacy against M. leprae infection induced in mice when compared to RMP (7). In this study, in vivo anli- M. leprae activity of KRM-1648 was evaluated in combination with DDS and CFZ.
MATERIALS AND METHODS
Special agents. KRM-1648, CFZ, and DDS were obtained from Kaneka Corporation, Hyogo, Japan; Ciba Geigy Co., Tokyo, Japan, and Wako Pure Chemical Ind., Osaka, Japan, respectively.
In vitro anti- M. leprae activity. Drug susceptibility testing using the BACTEC 460 TB System (Bccton Dickinson, Towson, Maryland, U.S.A.) was performed according to Franzblau (2), with some modifications. The inoculum was prepared from the foot pads of M. Ieprae -infected BALB/c nude mice by homogenizing the infected tissue with a glass homogenizcr in RPMI 1640 medium containing fetal bovine scrum. Cell debris were removed by subsequent centrifugation at 150 x g for 5 min. The resultant bacterial suspension was mixed with an equal volume of 4% NaOH. After dilution with 2 volumes of distilled water, the organisms were collected by centrifugation at 1500 x g for 20 min, washed twice with RPMI 1640 medium, and finally suspended in Tween 80-free 7H9 medium. KRM-1648, DDS, and CFZ were dissolved in ethanol at a concentration of 2 mg/ml and diluted to 80 µ g/ml in Tween 80-free 7H9 medium. BACTEC 12B medium (4 ml; without PANTA, containing 14C-palmitic acid) was inoculated with 0.1 ml of M. leprae suspension (108/ml) and 0.1 ml of diluted drug solution. Then, the air space of the BACTEC 12B vial was flushed out with 5% C0 2air and the vial incubated at 33ºC without agitation for up to 4 weeks. The growth index (GI) was read on days 4, 11, 18, and 27.
Experimental infection. M. leprae Thai-53 was harvested from the infected foot pads of BALB/c nude mice, and the bacterial suspension was prepared as follows. The infected foot pads were homogenized in Hanks' balanced salt solution containing 5% fetal bovine serum and ccntrifuged at 150 x g for 5 min. The upper layer was carefully removed. Bacilli were collected by reccntrifugation of the upper layer at 1500 x g for 15 min. The bacterial suspension was reccntrifuged at 150 x g for 5 min, and the upper layer was used as an inoculum for experimental infection. Female BALB/c nude mice (5 weeks old) were infected subcutaneously with 1 x 106 of M. leprae into the left hindfoot pad. Drugs were emulsified in 0.1 ml of 2.5% gum arabic-0.1% Twecn 80 solution by grinding with a mortar and pestle, and given by gavage once daily six times per week from day 31 to 80. All mice were observed for foot pad swelling. After 360 days the mice were killed, and the number of acid-fast bacilli in the left hindfoot pad was measured according to the method of Shepard (5). Statistical calculation was done by using Student's t test.
RESULTS AND DISCUSSION
Table 1 shows in vitro anti- M. leprae activities of KRM-1648, CFZ, DDS, and their combinations measured by the BACTEC 460 TB System. KRM-1648 (0.01 µ g/ml), CFZ (0.5 µ g/ml), and DDS (2.0 µ g/ml) exhibited a significant anti- M. leprae activity, reducing GI values by 78%, 30%, and 35% by day 18, respectively. Combinations of KRM with either CFZ, DDS or both caused a slight increase in the efficacy. Although the increase was not statistically significant, the same tendency was repeatedly found in the other two experiments.
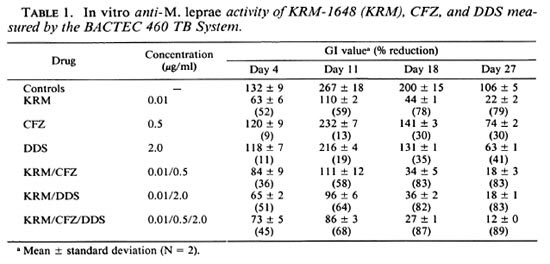
Table 2 shows the therapeutic efficacy of KRM-1648 alone and in combination with CFZ, DDS, or both against M. leprae infection induced in mice. In vivo anti- M. leprae activity of KRM-1648 (0.001 mg/ mouse) was intensified by the combined use of other agents, such as CFZ (0.1 mg/mouse) and DDS (0.2 mg/mouse), when compared to the efficacy of each drug alone. Administration of KRM-1648, CFZ, and DDS alone caused a 1.9-, 2.6-, and 2.2-log decrease in the number of leprosy bacilli recovered after 360 days, respectively, when compared to the bacilli in the control mice. The combinations of KRM-1648 + CFZ, KRM-1648 + DDS, and KRM-1648 + CFZ + DDS caused a 3.7-, 3.2-, and 4.4-log decrease, respectively. Although the therapeutic efficacies of KRM-1648 + DDS and KRM-1648 + CFZ + DDS were significantly higher than those of each drug alone (p < 0.025), the difference between KRM1648 + CFZ and CFZ alone was not significant.
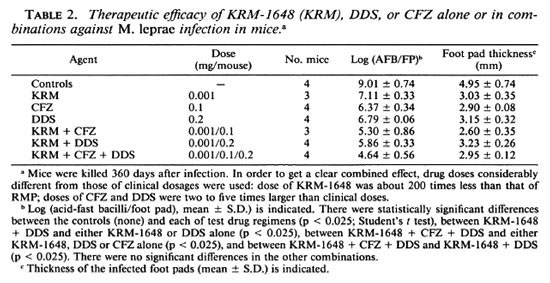
These findings demonstrated that in vivo anti- M. leprae activity of KRM-1648 (0.001 mg/mouse) can be enhanced when combined with other agents, such as CFZ (0.1 mg/mouse) and DDS (0.2 mg/mouse), as compared to the efficacy of each drug alone, although the combined effect of KRM-1648 with CFZ was not significant compared to that of CFZ alone. In separate experiments, KRM-1648 was found to exert the same level of inhibitory activity against bacterial RNA polymerase as that of RMP, thereby indicating the possibility that its permeability into the cell membrane was much improved from that of RMP (Fujii, et al , manuscript in preparation). Although more detailed studies using larger numbers of mice for each regimen are still needed to give a conclusion, the present data suggest that KRM-1648 would be effective in multidrug regimens for the clinical control of bacilliferous leprosy patients. KRM-1648 possessing much more potent antileprosy activity in vivo (7) may be preferable to RMP in multidrug regimens for clinical control of leprosy patients, given that KRM-1648 has comparable toxicity (Hidaka, T., et al , personal communication) and pharmacokinetics (9, 11). Although plasma and liver levels of KRM were 10- to 50-fold lower than those of RMP, its lung and spleen levels were equal to or higher than those of RMP (11). In addition, KRM-1648 showed a prolonged elimination from organs including the lungs, liver, spleen, and kidneys when compared to rifabutin, another new rifamycin derivative.
In the present study, the dose of KRM1648 was much lower than the equivalent clinical dosages because KRM-1648 caused complete inhibition of in vivo growth of M. leprae when given to mice at doses equivalent to the clinical dosages for rifamycins (3). This might cause some artificial features of the combined efficacy of this drug with other antileprosy agents. Therefore, it is necessary to carry out another experiment using clinical dosages of KRM-1648 in an M. leprae infection model where chemotherapy is given to infected animals possessing sufficiently high levels of bacterial loads in the foot pads.
In our previous study (6), it was found that the in vivo antileprosy activity of ofloxacin (OFLX) (3 mg/mouse) was significantly improved by combination with either RMP (0.01 mg/mouse) or DDS (0.2 mg/mouse), as compared to the efficacy of each drug alone. In one experiment, OFLX alone, RMP alone, and OFLX + RMP decreased the number of leprosy bacilli recovered after 365 days by 1.6-, 2.0-, and 3.3log units, respectively. In another experiment, OFLX alone, DDS alone, and OFLX + DDS decreased the number of leprosy bacilli recovered after 350 days by 0.9-, 1.0-, and 1.8-log units, respectively. Therefore, it seems that some new quinolones with appreciable in vivo anti- M. leprae activity, such as OFLX and sparfloxacin, may be useful in multidrug regimens containing a rifamycin for treating leprosy patients.
As previously reported (4, 8, 9) , KRM-1648 possesses much more potent in vitro and in vivo antimycobacterial activities than RMP. In addition, we observed significantly higher in vivo antileprosy activity of KRM-1648 compared to that of RMP (7). Therefore, it is possible that KRM-1648 may exert a better therapeutic efficacy against the M. leprae infection when used in MDT instead of RMP. The present study indicated that KRM-1648 displayed a combined therapeutic efficacy with DDS and CFZ, but this study did not make a direct comparison of the efficacies of KRM-1648 and RMP. Therefore, further detailed in vivo studies are needed both to evaluate the activity of KRM in combination with other antileprosy drugs and to compare its efficacy with that of RMP before clinical application of this drug for treatment of leprosy patients.
Acknowledgment. This study was supported by the Sasakawa Memorial Health Foundation and in part by the U.S.-Japan Coopérative Medical Science Program.
We are grateful to Dr. K. Kosaka for donating M. leprae -infected foot pads and to the Kancka Corporation and Ciba Geigy Co. for providing KRM-1648 and clofazimine, respectively.
REFERENCES
1. EMORI, M., SAITO, H., SATO, K., TOMIOKA, H., SETOGAWA, T. and HIDAKA, T. Therapeutic efficacy of benzoxazinorifamycin KRM-1648 against experimental Mycobacterium avium infection induced in rabbits. Antimicrob. Agents Chemother. 37(1993)722-728.
2. FRANZBLAU, S. G. Drug susceptibility testing of Mycobacterium leprae in the BACTEC 460 System. Antimicrob. Agents Chemother. 33(1989)2115-2117.
3. KATOCH, K., RAMU, G., RAMANATHAN, U., SENGUPTA, U., SREEVASTA, SHARMA, V. D., Sm-VANNAVAR, C. T. and KATOCH, V. M. Results of a modified WHO regimen in highly bacillifcrous BL/LL patients. Int. J. Lepr. 57(1989)451-457.
4. SAITO, H., TOMIOKA, H., SATO, K., EMORI, M., YAMANE, T., YAMASHITA, K., HOSOE, K. and HIDAKA, T. In vitro antimycobacterial activities of newly synthesized benzoxazinorifamycins. Antimicrob. Agents Chemother. 35(1991)542-547.
5. SHEPARD, C. C. The experimental disease that follows the injection of human leprosy bacilli into foot pads of mice. J. Exp. Med. 112(1960)445-454.
6. TOMIOKA, H. and SAITO, H. Therapeutic efficacy of some quinolones and a combination of ofloxacin with rifampin against Mycobacterium leprae infection induced in athymic nude mice. Int. J. Lepr. 61(1993)250-254.
7. TOMIOKA, H. and SAITO, H. In vivo antileprosy activity of the newly synthesized benzoxazinorifamycin, KRM-1648. Int. J. Lepr. 61(1993)255-258.
8. TOMIOKA, H., SAITO, H., FUJII, K., SATO, K. and HIDAKA, T. In vitro antimicrobial activity of benzoxazinorifamycin, KRM-1648, against Mycobacterium avium complex, determined by the radiometric method. Antimicrob. Agents Chemother. 37(1993)67-70.
9. TOMIOKA, H., SAITO, H., SATO, K., YAMANE, T., YAMASHITA, K., HOSOE, K., FUJII, K. and HIDAKA, T. Chemotherapeutic efficacy of a newly synthesized benzoxazinorifamycin, KRM-1648, against Mycobacterium avium complex infection induced in mice. Antimicrob. Agents Chemother. 36(1992)387-393.
10. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
11. YAMANE, T., HASHIZUME, T., YAMASHITA, K., KONISHI, E., HOSOE, K., HIDAKA, T., WATANABE, K., KAWAHARADA, H., YAMAMOTO, T. and KUZE, F. Synthesis and biological activity of 3'-hydroxy5' aminobenzoxazinorifamycin derivatives. Chem. Pharm. Bull. (Tokyo) 41(1993)148-155.
1. M.D., Ph.D., Professor; Department of Microbiology and Immunology.
2. Ph.D., Associate Professor; Department of Microbiology and Immunology.
3. Ph.D., Instructor; Department of Microbiology and Immunology.
4. M.D., Ph.D., Associate Professor, Department of Dermatology, Shimanc Medical University, Izumo 693, Japan.
Received for publication on 14 June 1993.
Accepted for publication in revised form on 16 November 1993.