- Volume 62 , Number 1
- Page: 48–54
IgG response to purified 65- and 70-kDa mycobacterial heat shock proteins and to antigen 85 in leprosy
ABSTRACT
IgG antibody response to mycobacterial heat-shock proteins (hsp) (the 70-kDa antigen f rom Mycobacterium tuberculosis and M. bovis BCG; the 65-kDa antigen f rom M. leprae and M. bovis BCG) and to the fibronectin-binding antigen 85 f rom M. bovis BCG was analyzed in a dot-blot assay in plasma f rom leprosy patients and their contacts. Most plasma-whatever the status of the subjects-reacted to the hsp 70; 8 of 9 (89%) of paucibacillary patients recognized the 65 mycobacterial hsp but only 2 of 9 (22%) recognized the antigen 85. In contrast, 12 of 12 (100%) of multibacillary patients reacted with the antigen 85 and only 4 of 12 (33%) reacted to the hsp 65 f rom M. leprae . On the one hand, 7 of 25 (28%) of the lepromin-positive contacts and 2 of 9 (22%) of the lepromin-negative contacts recognized the antigen 85. On the other hand, 11 of 25 (44%) of the lepromin-positive contacts but only 1 of 9 (11%) of the lepromin-negative contacts reacted to the hsp65 f rom M. leprae . Finally, very few (10%) of the lepromin-positive controls showed a positive reaction to the M. leprae 65-kDa antigen, the BCG 65-kDa antigen, and the 85-kDa antigen of BCG. Thus, differences in binding to the hsp65 f rom M. leprae and to antigen 85 could be helpful in distinguishing different forms of the disease.RÉSUMÉ
Les réponses d'anticorps IgG aux "heat-shock" protéines mycobactériennes (hsp) l'antigène de 70-kDa de Mycobacterium tuberculosis et du BCG de M. bovis ; l'antigène de 65-kDa de M. leprae et du BCG de M. bovis ) et à l'antigène 85 liant la fibronectine provenant du BCG de M. bovis a été analysée par "dot-blot" sur du plasma de patients lépreux et leurs contacts. La plus grande partie du plasma-quel que soit le statut des sujets-réagissait au hsp 70; huit des neuf patients paucibacillaires (89%) ont reconnu le hsp mycobacterien 65, mais seulement duex des neuf (22%) ont reconnu l'antigène 85. Par contraste, 12 des 12 patients multibacillaires (100%) ont réagi avec l'antigène 85 et seulement 4 sur 12 (33%) ont réagi au hsp 65 provenant de M. leprae . D'une part, 7 des 25 (28%) des contacts positifs à la lépromine et 2 des 9 (22%) des contacts négatifs à la lépromine ont reconnu l'antigène 85. D'autre part, 11 des 25 (44%) des contacts positifs à la lépromine, mais seulement 1 des 9 (11%) des contacts négatifs à la lépromine ont réagi au hsp 65 provenant de Al. leprae. Finalement, très peu (10%) des témoins positifs à la lépromine ont montré une réaction positive à l'antigène de 65 kDa de At. leprae, à l'antigène de 65 kD du BCG, et à l'antigène de 85 kD du BCG. Les différences dans la liaison au hsp 65 de M. leprae et à l'antigène 85 pourraient aider à faire la distinction entre différentes formes de la maladie.RESUMEN
Se analizó la respuesta en anticuerpos IgG contra las proteínas de choque térmico de 70 kD de Mycobacterium tuberculosis y M. bovis , BCG, contra la proteína de 65 kD de M. leprae y M. bovis BCG, y contra el antigeno 85 enlazante de la fibronectina, en cl plasma de pacientes con lepra y de sus contactos. La mayoría de lo plasmas-independientemente del estado de los pacientes-reaccionaron con la proteína de 65 kD pero sólo 2 de 9 (22%) reconocieron al antígeno de 85 kD. En contraste, 12 de 12 (100%) de los pacientes multibacilares reaccionaron con el antígeno 85 y sólo 4 de 12 (33%) reaccionaron con la proteína de 65 kD del M. leprae . Siete de 25 (28%) contactos lepromino-positivos y 2 de 9 (22%) lepromino-negativos reconocieron al antígeno 85. Por otro lado, 11 de 25 (44%) contactos lepromino-positivos y sólo 1 de 9 (11%) contactos lepromino-negativos reaccionaron con la proteína de 65 kD del M. leprae . Finalmente, muy pocos de los controles lepromino-positivos (10%) mostraron una reacción positiva con el antígeno de 65 kD de M. leprae , con el antígeno de 65 kD del BCG, y con el antígeno de 85 kD del BCG. Así, las diferencias en el reconocimiento de la proteína de 65 kD del M. leprae y del antígeno 85, podrían ser de utilidad para distinguir las diferentes formas de la enfermedad.One aim of epidemiological studies in leprosy is the early diagnosis of subclinical infection in patients infected with Mycobacterium leprae . If antibodies do not confer protective immunity in leprosy, they might be used as a marker of M. leprae infection. For several years the number of assays that may detect antibodies to M. leprae has been expanding. Antibodies directed against phenolic glycolipid-I (PGL-I) and its derivatives specific for M. leprae (4) and lipoarabinomannan (LAM) (15) and against defined molecules, particularly the 65-(10), 36-(11), 35-(10, 18) and 18-(17) kDa antigens from M. leprae , have been well documented. However, only a minority of paucibacillary patients have antibodies to these antigens. Furthermore, in follow-up studies the presence of antibodies to PGL-I was not predictive of the clinical outcome (19).
A number of defined molecules, obtained from mycobacteria either through DNA technology or by classical purification procedures, are now available in large amounts. A number of these antigens have been defined as heat-shock proteins (hsp): the 65-and 70-kDa molecules with chaperoninc functions (9) are known to play a role in the immune response to many bacterial or parasitic pathogens. Another group of antigens are secreted antigens, a major component being antigen 85 (Ag85), a widely crossre active antigen found in all mycobacterial species which bind to fibronectin (1). Both hsp and Ag85 carry B-cell epitopes (3, 10, 13) but the distribution of specific antibodies across the leprosy spectrum is not yet well established.
In this paper we have examined the antibody responses to these proteins (the 70kDa from M. tuberculosis and M. bovis BCG; the 65 kDa from M. leprae and M. bovis BCG; antigen 85 from M. bovis BCG) in an attempt to identify molecule(s) that could eventually be used in a serodiagnostic assay.
MATERIALS AND METHODS
Subjects. Twenty-one patients classified according to Ridley-Jopling criteria (l6) were included in this study. All patients were monitored in the Institut de Leprologie Appliquée de Dakar, Senegal. Twelve were classified as multibacillary patients (6 BL, 6 LL) and nine were classified as paucibacillary patients (5 BT, 4 TT). All patients were studied before treatment. Ten healthy lepromin-positive and eight healthy lepromin-negative controls were recruited at the Institut Pasteur de Dakar and included in this study. Finally, 34 household contacts of leprosy patients, living in a rehabilitation village together with their family members with leprosy were studied; 25 of these contacts were lepromin-positive and 9 were lepromin-negative.
Antigens. Antigen 85 (Ag85) was purified from culture filtrate of M. bovis BCG by sequential chromatography on Phenyl-Sepharose and DEAE-Sephacel as previously described (7). Purified recombinant 65 kDa from M. leprae (ML65) and M. bovis BCG (BCG65) (homologous to GroEL/ hsp60), recombinant 70 kDa from M. tuberculosis (TB70) (homologous to DNA K/hsp70) were provided by Dr. J. van Embden through the WHO/TDR/IMMLEP special program. Briefly, these proteins were obtained from heat-induced (42ºC) Escherichia coli K12 which carries plasmid pRIB 13001, pZW1003 and pKAM2101, respectively, for the hsp65 from M. bovis BCG and M. leprae and the hsp70 from M. tuberculosis . After lysis of the cells, the recombinant proteins were precipitated with ammonium sulfate, dialyzed, and further purified by DEAE column chromatography. The 70-kDA protein from M. tuberculosis was then purified by ATP affinity chromatography. The affinity chromatography purified 70-kDa from M. bovis BCG (BCG 70) was a generous gift from Prof. A. Basten (Sydney, New South Wales, Australia) and was purified as previously described using the monoclonal antibody L22 (3).
Lepromin test. The Mitsuda reaction was examined with lepromin (4 X 106 acid-fast bacilli) provided by the World Health Organization (WHO; R. J. W. Rees, National Institute of Medical Research, London). Induration was measured after 4 weeks (day 28), and the test was considered positive when the diameter of the papule was > 3 mm.
Dot-blot enzyme-linked immunoassay analysis. Each antigen (BCG65, ML65, BCG70, TB70 and Ag85) was immobilized at 0.5 µ g on a 0.45- µ m pore size nitrocellulose paper (NC) (Schleicher & Schull, Danel, Germany). The NC paper was cut into strips each having 5 dots, immersed in blocking reagent [5% bovine albumin in phosphate buffer (PBS)] for 1 hr at room temperature. Then NC strips were incubated overnight with human plasma diluted 1/100 in PBS 0.05% Tween 20 (PBS-T), rinsed 3 times in PBS-T, and incubated for another 1 hr with peroxidase goat anti-human IgG immunoglobulin (Sanofi Diagnostics Pasteur, Marnes la Coquette, France) diluted 1/1000 in PBS-T. After rinsing, the NC strips were stained by the addition of diamino-benzidine in the presence of H2O2.
To quantify the intensity of staining of the dots, a GS-300 densitometer (Hoeffer Scientific Instruments, San Francisco, California, U.S.A.) was used. Data in mV were analyzed with the GS-370 Data System. A positive reaction was defined as a result greater than the mean of results from healthy lepromin-negative controls plus three standard deviations.
RESULTS
IgG reactivity to the 70-kDa hsp. As shown in The Table, the majority of leprosy patients and their contacts reacted to both of the 70-kDa molecules from M. tuberculosis and M. bovis BCG. Reactivity of plasma from multibacillary and paucibacillary patients was of comparable magnitude. Plasma samples from contacts and controls with a lepromin-positive reaction (lepromin-positive controls and contacts) were more reactive than plasmas from leprominnegative contacts and controls (Fig. 1).
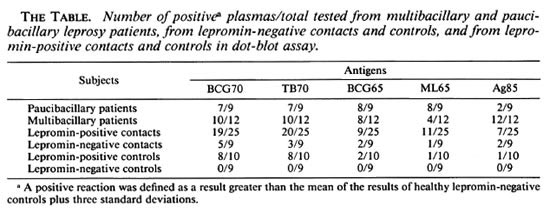
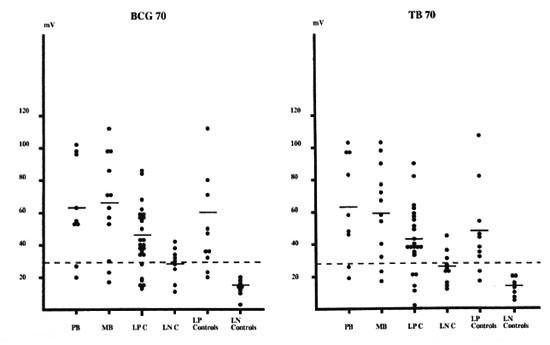
Fig. 1. IgG antibody response against BCG 70 and TB70. Shown are intensity in mV for paucibacillary (PB, N = 9) and multibacillary (MB, N = 12) leprosy patients; lepromin-positive (LPC, N = 25) and lepromin-negative (LNC, N = 9) leprosy contacts; healthy lepromin-positive (LP Controls, N = 10) and lepromin-negative (LN Controls, N = 8) controls;  = mean of results from healthy lepromin-negative controls plus three standard deviations.
= mean of results from healthy lepromin-negative controls plus three standard deviations.
IgG reactivity to the 65-kDa hsp. As for reactivity against the 70-kDa molecules, the majority of the plasma samples from paucibacillary patients reacted to the hsp 65kDa from M. leprae and M. bovis BCG (The Table). In contrast, only a small number of plasma samples from multibacillary patients and leprosy contacts reacted to these molecules.
Plasma from lepromin-positive subjects (paucibacillary patients and lepromin-positive contacts) reacted more strongly to the 65-kDa hsp than did plasma from lepromin-negative subjects (multibacillary patients and lepromin-negative contacts) (Fig. 2). This was particularly pronounced with ML65.
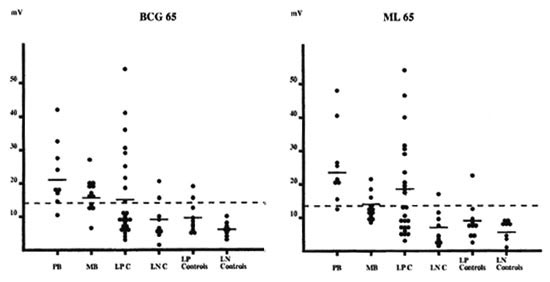
Fig. 2. IgG antibody response against BCG65 and ML65 (for further details, see The Table).
IgG reactivity to Ag85. Twelve out of the 12 multibacillary patients but only 2 out of the 9 paucibacillary patients showed a positive IgG response to Ag85. A very few leprosy contacts reacted with this molecule (The Table). Reactivity was higher in plasma from multibacillary patients than from other subjects (Fig. 3).
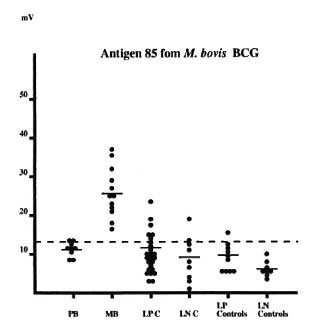
Fig. 3. IgG antibody response against Ag85 (for further details, see The Table).
DISCUSSION
In this study we have demonstrated the majority of plasma samples from leprosy patients and their contacts react to the hsp70, whatever the status of their lepromin reactivity. The high degree of hsp70 staining of plasma from leprosy patients and their contacts might possibly be explained by interleukin 6 (IL-6) secretion induced by these molecules. IL-6 has plciotropic activities incuding terminal differentiation of B cells (BSF-2 activity) (20). Recently, we have shown that in leprosy patients and their contacts the 70-kDa hsp can function as a powerful IL-6 inducer (13). In parallel with malaria, in which IgG antibodies to the Plasmodium falciparum -hsp70 have been detected in more than 70% of adults and were found to correlate with the exposure to Plasmodium (2), IgG antibodies to mycobacterial 70 hsp might be a marker of exposure to mycobacteria.
The 65-kDa antigen is considered to be the most important component of M. leprae recognized by multibacillary patients. Indeed, Levis, et al. have demonstrated that serum from multibacillary patients shows higher antibody levels to the 65-kDa molecules than serum from paucibacillary patients (14). However, in our present work, we have demonstrated that plasma from lepromin-positive individuals (paucibacillary patients and healthy lepromin-positive contacts) reacted more strongly to the hsp65 than plasma from lepromin-negative individuals (multibacillary patients and healthy lepromin-negative contacts), particularly to ML65. This is in accordance with data from Das, et al. which demonstrated that plasma from tuberculoid patients reacted more strongly to BCG65 hsp than lepromatous leprosy patients in an ELISA and in a Western blot analysis (6). However, in contrast with the work reported by Das, a significant proportion (11 of 34) of contacts bound to the 65 hsp in dot-blot assay. The low level of leprosy endemicity in Spain or in Germany, where the contacts for the Dutch work were collected, as compared to the leprosy endemicity in Africa may explain the discrepancies observed between these reports.
Finally, all multibacillary patients but only 2 out of the 9 paucibacillary patients reacted to Ag85. Ag85 is a protein family with three members, 85A, 85B, 85C, encoded by three distinct genes with respective molecular weights of 32, 30 and 33 kDa (5, 22). In this present study, the complete Ag85 complex (85 A + 85 B + 85 C components) was used, and it is difficult to know which of these components is immunodominant in the dot-blot assays. Since reactivity against the Ag85 of the two positive plasmas from paucibacillary patients is very close to the limit of the positivity (Fig. 3), we hypothesize that these plasmas reacted to the Ag85A component of the complex. Indeed, we have shown previously, using an isoelectric technique to separate the components of the complex, that plasma samples from all multibacillary patients and the majority of paucibacilary leprosy patients react to the Ag85A component and that only plasma from multibacillary patients also react to the Ag85B component (8, 21)- Therefore, the sensitivity of the anti-complex 85 (A + B + C) is insufficient to be used as diagnosis.
Recently, we compared purified 65-kDa and 70-kDa hsp to the purified Ag85 and found that Ag85 is the most powerful T-cell antigen in M. leprae infection, eliciting a strong proliferative response and interfcron-gamma(IFN- γ ) production in all (100%) paucibacillary patients and in leprominpositive contacts and controls (12). This dichotomy between T-cell reactivity and humoral response toward antigen and M. leprae is in accordance with the cytokine secretion profile found in leprosy skin biopsies where IL-2 and IFN- γ mRNA (TH1 profile) were found in tuberculoid lesions and IL-4 and IL-5 mRNA (TH2 profile) were found in lepromatous lesions (24). However, this inverse correlation between antibody response and T-cell stimulation is not universal: we demonstrated in this present work significant antibody levels toward the hsp65 in plasma from some paucibacillary patients that mount a T-cell proliferation toward the 65 hsp.
The aim of this study was to find a serological assay capable of detecting antibodies specific for leprosy: the hsp proteins (65- and 70-kDa hsp) and Ag85 are clearly not good candidates for such an assay (The Table), confirming recent data with the 65(10) and 18-kDa hsp (17). However, differential reactivity to hsp65 and Ag85 could be used perhaps as an aid to distinguish the paucibacillary and multibacillary forms of leprosy. Indeed, multibacillary and paucibacillary patients recognized Ag85 and hsp65, respectively. Recently, cloning and expression of the genes of Ag85 from M. leprae have been reported (De Mendonca Lima, personal communication). Comparison of components of Ag85 and hsp65 from M. leprae will be undertaken in the near future to examine whether this differential reactivity at the two poles of the disease can be confirmed.
Acknowledgment. The authors would like to thank Dr. J. van Embdcn and Prof. A. Basten for providing the purified heat-shock proteins. This investigation received financial support from the UNDP/World Bank/ WHO Special Programme for Research and Training in Tropical Diseases, from the Damiaanaktie (Brussels), and the Association Raoul Follereau (Paris). AD is a fellow of the Foundation Erasme 1992-1993.
REFERENCES
1. ABOU-ZEID, C, RATLIFF, T. L., WIKER, H. G., HARBOE, M., BENNEDSEN, J. and ROOK, A. W. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect. Immun. 56(1988)3046-3051.
2. BEHR, C, SARTHOU, J. L., ROGIER, C, TRAPE, J.-F., HYUNH QUAN DAT, M., MICHEL, J-C , ARIBOT, G., DIEYE, A., CLAVERIE, J.-M., DRUILHE, P. and DUBOIS, P. Antibodies and reactive T cells against the malaria heat-shock protein Pf 72/Hsp70-1 and derived peptides in individuals continuously exposed to Plasmodium falciparum . J. Immunol. 149(1992)3321-3330.
3. BRITTON, W. J., GARSIA, R. J., HELLQVIST, L., WATSON, J. D. and BASTEN, A. The characterization and immunorcactivity of a 70 kD protein common to Mycobacterium leprae and Mycobacterium bovis (BCG). Lepr. Rev. 57 Suppl. 2(1986)67-75.
4. CHANTEAU, S., CARTEL, J.-L., ROUX, J., PLICHART, R. and BACH, M.-A. Comparison of synthetic antigens for detecting antibodies to phenolic glycolipid in patients with leprosy and their household contacts. J. Infect. Dis. 157(1988)770-776.
5. CONTENT, J., de la CUVELLERIE, A., DE WIT, L., VINCENT-LEVY-FREBAULT, V., OOMS, J. and DE BRYUN, J. The genes coding for the antigen 85 complexes of Mycobacterium tuberculosis and Mycobacterium bovis BCG are members of a gene family: cloning, sequence determination and genomic organization of the gene coding for antigen 85-C of M. tuberculosis . Infect. Immun. 59(1991)3205-3212.
6. DAS, P. K., RAMBUKKANA, A., BAAS, J. G., GROO-THUIS, D. G. and HALPERIN, M. Enzyme-linked immunosorbent assay for distinguishing serological responses of lepromatous and tuberculoid leprosies to the 29/33-kilodalton doublet and 64-kilodalton antigens of' Mycobacterium tuberculosis . J. Clin. Microbiol. 28(1990)379-382.
7. DE BRUYN, J., HUYGEN, K., BOSMANS, R., FAU-VILLE, M., LIPPENS, R., VAN VOOREN, J. P., FAL-MAGNE, P., WECKX, M., WlKER, H. G., HARBOE, M. and TURNEER, M. Purification, characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb. Pathogen. 2(1987)351-366.
8. DROWART, A., LAUNOIS, P., DE COCK, M., HUYGEN, K., DE BRUYN, J., YERNAULT, J. C. and VAN VOOREN, J. P. An isoelectric focusing method for the study of the humoral response against the antigen 85 complex of Mycobacterium bovis BCG in different forms of leprosy. J. Immunol. Methods 145(1991)223-228.
9. ELLIS, R. J. The molecular chaperon concept. Sem. Cell. Biol. 1(1990)1-9.
10. KHAN, M. B., DESHPANDE, R. G., DAVIDSON, S. K. and NAVALKAR, R. G. Sero-immunoreactivity of cloned protein antigens of Mycobacterium leprae . Int. J. Lepr. 60(1992)195-200.
11. KLATSER, P. R., DE WIT, M. Y. and KOLK, A. H. J. An ELISA inhibition test using monoclonal antibody for the serology of leprosy. Clin. Exp. Immunol. 62(1985)468-473.
12. LAUNOIS, P., N'DIAYE NIANG, M., SARTHOU, J. L., RIVIER, J. L., DROWART, A., VAN VOOREN, J. P., MILLAN, J. and HUYGEN, K. T-cell stimulation with purified mycobacterial antigens in patients and healthy subjects infected with Mycobacterium leprae , secreted antigen 85 is another immunodominant antigen. Scand. J. Immunol. 38(1993)167-179.
13. LAUNOIS, P., VANDENBUSSCHE, P., N'DIAYE NIANG, M., DROWART, A., VAN VOOREN, J. P., SARTHOU, J. L., MILLAN, J. and HUYGEN, K. IL-6 production in response to purified mycobacterial heat-shock proteins and to antigen 85 in leprosy. Cell. Immunol. 148(1993)283-290.
14. LEVIS, W. R., MEEKER, H. C, SCULLER-LEVIS, G. B., GILLIS, T. P., MARINO, L. J. and ZABRISKIE, J. Serodiagnosis of leprosy: relationships between antibodies to Mycobacterium leprae phenolic glycolipid I and protein antigens. J. Clin. Microbiol. 24(1986)917-921.
15. MILLER, R. A., HARNISCH, J. B. and BUCHANAN, T. M. Antibodies to mycobacterial arabinomannan in leprosy: correlation with reactional states and variation during treatment. Int. J. Lepr. 52(1984)133-139.
16. RIDLEY, D. S. and JOPLING, W. M. Classification of leproys according to immunity; a five-grade system. Int. J. Lepr. 34(1966)255-273.
17. ROCHE, P. W., PRESTIDGE, R. L., WATSON, J. D. and BRITTON, W. J. Antibody responses to the 18 kDa protein of Mycobacterium leprae in leprosy and tuberculosis patients. Int. J. Lepr. 60(1992)201-207.
18. SINHA, S., SENGUPTA, U., RAMU, G. and IVANYI, J. Serological survey of leprosy and control subjects by a monoclonal antibody based assay. Int. J. Lepr. 53(1985)33-38.
19. ULRICH, M., SMITH, P. G., SAMPSON, C, ZUNIGA, M., CENTENO, M., GARCIA, V., MANRIQUE, X., SALDAGO, A. and CONVIT, J. IgM antibodies to native phenolic glycolipid-I in contacts of leprosy patients in Venezuela: epidemiological observations and a prospective study of risk of leprosy. Int. J. Lepr. 59(1991)405-415.
20. VAN SNICK, J. Interleukin-6: an overview. Annu. Rev. Immunol. 8(1990)253-278.
21. VAN VOOREN, J. P., DROWART, A., DE BRUYN, J., LAUNOIS, P., MILLAN, J., DELAPORTE, E., DEVELOUX, M., YERNAULT, J. C. and HUYGEN, K. Humoral response against the 85A and 85B antigens of Mycobacterium bovis BCG in patients with leprosy and tuberculosis. J. Clin. Microbiol. 30(1992)1608-1611.
22. WIKER, H. G. and HARBOE, M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis . Microbiol. Rev. 56(1992)648-661.
23. WIKER, H. G., SLETTEN, K., NAGAI, S. and HARBOE, M. Evidence for three separate genes encoding the proteins of the mycobacterial antigen 85. Infect. Immun. 58(1990)272-274.
24. YAMAMURA, M., UYEMURA, K., DEANS, R. J., WEINBERG, K., REA, T. H., BLOOM, B. R. and MODLIN, R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254(1991)277-279.
1. M.D.;Institut Pasteur de Dakar, B. P. 220, Dakar, Senegal.
2. Biological Pharmacist, Institut Pasteur de Dakar, B. P. 220, Dakar, Senegal.
3. Biological Pharmacist, Immunologic, Institut Pasteur de Dakar, B. P. 220, Dakar, Senegal.
4. M.D.; Service de Pneumologie, Hôpital Erasme, Brussels, Belgium.
5. M.D., ULB, Service de Pneumologie, Hôpital Erasme, Brussels, Belgium.
6. M.D.;Institut de Leprologie Appliquée de Dakar, Dakar, Senegal.
7. M.D., Institut de Leprologie Appliquée de Dakar, Dakar, Senegal.
8. Ph.D., Department de Virologie, Institut Pasteur van Brabant, Brussels, Belgium.
Received for publication on 28 June 1993.
Accepted for publication in revised form on 8 October 1993.