- Volume 62 , Number 1
- Page: 1–9
An epidemiological study of leprosy infection by serology and polymerase chain reaction
ABSTRACT
A population-based study has been carried out in two adjacent villages in a highly leprosy-endemic area of South Sulawesi, Indonesia. The prevalence of clinical leprosy was 10.0 per 1000 inhabitants. A total of 1015 serum samples and 1228 nasal swab specimens were collected. IgM antibodies in blood to phenolic glycolipid-I (PGL-I) of Mycobacterium leprae were demonstrated by the gelatin particle agglutination test (MLPA) and by indirect ELISA (IgM-PGL). IgG antibodies to PGL-I (IgG-PGL) and lipoarabinomannan-B (IgG-LAM) were measured by indirect ELISA. The presence of M. leprae in nasal swab specimens was established by a polymerase chain reaction (PCR).The seropositivity rates in the population were 32% for MLPA, 30.8% for IgM-PGL, 6.7% for IgG-PGL, and 11.6% for IgG-LAM. Seropositivity rates for MLPA and IgM-PGL were highest in the younger age groups. There was no difference in seropositivity in any of the tests between household contacts of leprosy patients and noncontacts. The seropositivity rates in the MLPA and IgM-PGL were not randomly distributed among all households. The presence of M. leprae by PCR was demonstrated in 7.8% of the nasal swab specimens. No correlation was found between the results of the PCR and serology. This study indicates that M. leprae is widespread in the population, and that in endemic areas many individuals carry M. leprae in their nasal cavities without having obvious symptoms of leprosy.
RÉSUMÉ
Une étude a été réalisée dans la population de deux villages adjacents dans une région à haute endémicité lépreuse de Sud-Sulawesi en Indonésie. La prévalence de la lèpre clinique était de 10,0 pour 1000 habitants. Un total de 1015 échantillons de serum et 1228 prélèvements en provenance des fosses nasales a été collecté. La présence dans le sang d'anticorps IgM visà-vis du glycolipide phénolique I (PGL-I) de Mycobacterium leprae a été démontrée par le test d'agglutination de particules de gélatine (MPLA) et par ELISA indirect (IgM-PGL). Les anticorps IgG (IgG-PGL) et le lipoarabinomannan-B (IgG-LAM) ont été mesurés par ELISA indirect. La présence de M. leprae dans les prélèvements en provenance des fosses nasales a été établie par une réaction de polymerase en chaîne (PCR).Les taux de séropositivité observés dans la population étaient de 32% pour MPLA, 30, 8% pour IgM-PGL, 6, 7% pour IgG-PGL, et 1 1, 6% pour IgG-LAM. Les taux de séropositivité pour MPLA et IgM-PGL étaient les plus élevés dans les groupes d'âge jeunes. Il n'y avait pas de différence de séropositivité pour aucun des tests entre les contacts domiciliaires de patients lépreux et les noncontacts. Les taux de séropositivité pour le MPLA et l'IgM-PGL n'étaient pas distribués de manière aléatoire parmi l'ensemble des habitations. La présence de M. leprae à la PCR a été mise en évidence dans 7, 8% des prélèvements provenant des fosses nasales. On n'a pas trouvé de correlation entre les résultats de la PCR et la sérologie. Cette étude indique que M. leprae est largement répandu dans la population, et que dans les régions endémiques, de nombreuses personnes sont porteuses de M. leprae dans leurs cavités nasales sans avoir de symptômes évidents de lèpre.
RESUMEN
Se efectuó un estudio poblacional en dos comunidades vecinas de un área de Sulawesi del Sur (Indonesia) con alta endemia de lepra, en donde la prevalência de lepra clínica fue de 10 por 1000 habitantes. Se colectaron un total de 1015 muestras de suero y 1228 especímenes de exudado nasal. Se buscaron anticuerpos IgM contra el glicolípido fenólico I (PGL-I) de Mycobacterium leprae usando la técnica de aglutinación de partículas de gelatina (MLPA) y un ELISA indirecto (IgM-PGL). Los anticuerpos IgG anti PGL-I (IgG-PGL) y anti-lipoarabinomanana (IgG-LAM) se midieron usando un ELISA indirecto. La presencia de M. leprae en los especímenes de exudado nasal se estableció mediante una reacción en cadena de la polimcrasa (PCR).Los grados de seropositividad en la población fueron del 32% para MLPA, del 30.8% para IgM-PGL, del 6.7% para IgG-PGL, y del 11.6% para IgM-LAM. Los índices de seropositividad en las pruebas MLPAe IgM-PGL no estuvieron distribuidos aleatoriamente entre todos los contactos familiares. La presencia de M. leprae por PCR se demostró en el 7.8% de los especímenes de exudado nasal. No se encontró correlación entre los resultados por PCR y la scrología. Este estudio indica que M. leprae está ampliamente distribuido en la población y que en las áreas endémicas muchos individuos alojan al M. leprae en sus cavidades nasales sin tener los síntomas obvios de la lepra.
Although the prevalence of leprosy has declined worldwide over the past decades, the incidence has not declined in many developing countries (22). Apart from social stigma and economic loss, the sequelae of leprosy involve severe deformities. An estimated 2 to 3 million individuals are handicapped as a result of having ever contracted leprosy (18).
The generally accepted concept is that overt leprosy patients are the main source of transmission. If one accepts this view then a control strategy based on case finding and treatment would ultimately lead to the eradication of leprosy. However, the effectiveness of this approach in the reduction of leprosy incidence is difficult to assess (10).
Little is known about the distribution and transmission of infection and the factors leading to disease, mainly due to the inability to grow the causative organism of leprosy, Mycobacterium leprae .
The employment of serological assays based on the detection of antibodies to species-specific antigens of M. leprae have opened new possibilities to study M. leprae infection. It is generally assumed that antibody levels are associated with the intensity of exposure to M. leprae (21). It also has become clear that infection with M. leprae is more prevalent than disease. On the other hand, serological assays do not reflect all clinical infections, since the majority of paucibacillary patients do not elicit a detectable humoral response (2). In addition, follow-up studies have shown that new cases of leprosy are also found in the seronegative groups (21), suggesting that neither are all subclinical infections necessarily reflected by a serological response. Another limitation of serology may be that it cannot distinguish between past and present infection. With these provisos in mind, serology may still give valuable information on the extent of infection in a population and on the transmission of the infection. Assuming that the prevalence of seropositivity in a population roughly reflects exposure/infection rates, the effect of control measures may thus be evaluated upon repeated serological screening. The polymerase chain reaction (PCR) for the detection of M. leprae has given new opportunities in the study of the epidemiology of leprosy, since the presence of M. leprae DNA is proof of the presence of bacili (7, 13, 14).
South Sulawesi is an area with one of the highest prevalences of leprosy in Indonesia (5). The factors that determine such a high endemicity are not known. In order to get insight into such determinants it is first necessary to collect basic data on infection (carriage?) rates in the population in addition to data based on clinically diagnosed patients rates.
The use of PCR and serological assays, cither alone or in combination, could lead to a better understanding of the epidemiology of M. leprae infection. We performed a population-based study in this high endemic area in which the prevalence of antibodies to M. leprae antigens in serum was measured in conjunction with the presence of M. leprae in the nose as determined by PCR.
MATERIALS AND METHODS
Field study. Two isolated adjacent villages in a rural area of South Sulawesi, Indonesia, highly endemic for leprosy (5) were selected for the survey. The village of Bantimala had 1193 inhabitants and Tondongkura had 738. Multidrug therapy (MDT) had not yet been introduced in the area and the registered patients all received, often at irregular intervals, dapsonc monotherapy. The villages shared similar characteristics in terms of geographical, socioeconomic and cultural conditions. The population consisted mainly of subsistcnt farmers; cash in come was received from sales of surplus and from relatives working elsewhere. Most villagers were living in wooden houses builton stilts. The facilities for drinking water and sanitation were poor. Health care was provided by one health center in Bantimala and a small village health post in Tondongkura, both of which were staffed only by paramedical personnel.
Before the study was undertaken the villages were informed of the purpose of the study and consent was obtained from all participants. Only residents living for at least 3 months in the villages were included in the study.
Clinical examination was carried out by experienced leprosy workers and diagnosis based on the Ridley-Jopling classification (19) was confirmed by the provincial leprosy medical officer. The bacterial index (BI) was determined from slit-skin smears taken from all patients. Household contacts of patients were defined as those persons living in the same house as the index case.
Blood was collected from individuals between 5 and 65 years of age by venipuncture. Blood was left to clot and the serum was separated by centrifugation in the villages. Sodium azide (0.01%) was added to the sera, which were then stored at 4ºC and transported to the laboratory where they were kept refrigerated until analyzed.
Nasal swab specimens were collected and examined for the presence of M. leprae by a polymerase chain reaction (PCR) as described elsewhere (6).
Serological assays. The presence of antibodies to phenolic glycolipid I (PGL-I) was established in a simple gelatin particle agglutination test (MLPA Scrodia-leprac kit, Fujircbio, Japan) (15). This test will be referred to as MLPA.
In addition, the presence of IgM and IgG antibodies to PGL-I was measured in enzyme-linked immunosorbent assays (ELI-SAs) as described previously (2). In these assays, the semisynthetic trisaccharide analog, NT-P-BSA, of PGL-I was employed as antigen (12). These tests will be referred to as IgM-PGL and IgG-PGL, respectively. The presence of IgG antibodies to lipoarabinomannan B (LAM-B) from M. tuberculosis H37Ra, kindly provided by Dr. P. J. Brennan, Colorado State University, Fort Collins, Colorado, U.S.A., were demonstrated in an ELISA as described previously (8). This test will be referred to as IgG-LAM.
For each serum sample the optical density (OD) obtained in the ELISA with uncoated wells was subtracted from the value obtained with the antigen-coated wells. All serum samples were tested in duplicate and if the variance between the two wells was more than 10%, the scrum was rctested. The plateto-platc variation was controlled by using a standardized reference serum on each plate.
The positive threshold values were defined by using the 95-percentile of the OD values obtained with sera from nonendemic controls from Japan. This resulted in the following cut-ofT values: for the MLPA, a titer of 1:32; for IgM-PGL, an OD of 0.380; for IgG-PGL, an OD of 0.380; for IgG-LAM, an OD of 0.250.
PCR. Treatment of the swab specimens with lysis bufier and PCR was done as reported previously (6). Briefly, the specimens were subjected to PCR for the species-specific amplification of a fragment of the pra gene of M. leprae and subsequently were analyzed for the presence of a 531-bp amplification product by agarose gel electrophoresis followed by hybridization with a 1.0 Kb EcoRl fragment comprising the pra gene of M. leprae as a DNA probe. To determine if the sample contained inhibiting components that could have caused a negative result, samples in which a 531-bp fragment was not detected were submitted to a second PCR in which a 531-bp modified template was added to the reaction mixture. DNA was purified from the samples which were found to inhibit amplification of the modified template. These samples were run again in PCR. PCR reaction mixtures contained dUTP and uracil-DNA-glycosylasc to prevent false-positive reactions due to cross-contamination with amplified DNA. In each run, positive controls of chromosomal M. leprae DNA were included, as well as five negative controls without target DNA (i.e., lysis bufier). A specimen was considered positive when it revealed, with or without purification of DNA, a 531 -bp fragment by both agarose gel electrophoresis and subsequent hybridization. A sample was considered negative when it did not show amplification in the first PCR and did not inhibit the amplification of the modified template. If a sample inhibited the amplification of the modified template, even after purification, we could not determine whether it contained M. leprae DNA or not. A total of 37 samples (2.9%) were excluded from analysis as being uninterpretable.
Data analysis. All data were entered on a personal computer and analyzed using Epi- Info version 5. Analysis of variance methods were applied as indicated in the text. All probabilities presented are two-tailed, unless otherwise stated.
To examine whether the distribution of scropositivity was equal among all households, we compared the observed and expected number of seropositives among households in a goodness-of-fit test ( Σ [(0-E)2/E]). The expected frequency of scropositivity in households was calculated for each household size using the formula (p + q)n, whereby p = overall scropositivity rate, q = p - 1 and n = number of persons tested in the household. The expected number of households with each frequency of scropositivity was then obtained by multiplication of the expected frequency with the number of households in each size category.
RESULTS
Survey. A total of 954 males and 977 females were registered in the two villages. Figure 1 illustrates the age and sex distribution of the registered and serologically tested population. In the 20-50 age cohort the sex ratio was 0.8, reflecting that a number of males had left the village in search of work elsewhere.
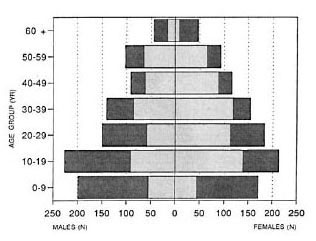
Fig. 1. Distribution of the registered and tested population according to age and sex.  =registered;
=registered;  = tested (tested group comprised only persons be-tween 5 and 65 years of age).
= tested (tested group comprised only persons be-tween 5 and 65 years of age).
A total of 1302 persons were clinically examined, representing 67.4% of the registered population. The male : female ratio in the examined population was 0.83 compared to 0.98 in the registered population.
During the survey a total of 13 leprosy patients were diagnosed, 10 new and 3 old patients, giving a prevalence in these two villages of 10/1000. Of the 13 leprosy patients three were females (23.1%), which is a significantly lower prevalence of leprosy in females than in males in this population (Fisher's exact test, p < 0.01). The clinical classification of 4 patients was BL (Bis of the slit-skin smears ranging from 1.0 to 3.5); 3 were diagnosed as BT and 6 as TT. The age of the patients ranged between 16 and 55 years.
A total of 1015 sera were examined for the presence of antibodies using the four different serological assays, representing 78.0% of the number of persons clinically examined.
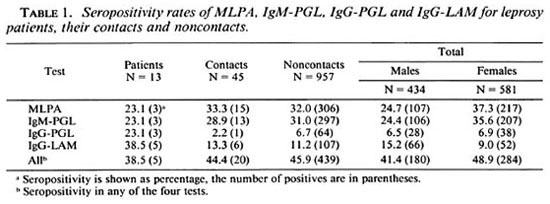
Serology. The seropositivity rates of the four tests for leprosy patients, their contacts and noncontacts are shown in Table 1. The three multibacillary patients from whom serum was obtained were seropositive, while four paucibacillary patients were negative in all tests. Two more paucibacillary patients were positive in the IgG-LAM.
The seropositivity rates obtained with all assays did not differ between the household contacts and the nonhousehold contacts (Chi-squared test, p > 0.05). Therefore, infurther analysis no distinction was made be-tween contacts and noncontacts.
The overall seropositivity rates were32.0% for MLPA, 30.8% for IgM-PGL, 6.7%for IgG-PGL, and 11.6% for IgG-LAM.
When the seropositivities of the differentassays were combined, the overall percent-age of persons being seropositive in any ofthe four tests was 45.8%.
The seropositivity rates of MLPA and IgM-PGL were significantly higher in females compared to males (Mantel-Haenszel summary Chi-squared test stratified for age,MLPA = p < 0.001, IgM-PGL = p < 0.001). Seropositivity was found to be higher in males than in females with IgG-LAM (Mantel-Haenszel summary Chi-squared test stratified for age, p < 0.001). With IgG-PGL the difference in seropositivity between males and females was small and insignificant. However, the mean test values (OD or titer) of the tested persons was not different in males and females for any of these tests (Kruskal-Wallis test for IgM-PGL, p = 0.22; Chi-squared test for trendfor MLPA, p = 0.17; result not shown).
Figure 2 shows the seropositivity rates obtained with the four assays according to the age of the tested population. The prevalence of MLPA and IgM-PGL seropositivity was highest in the younger age groups and declined with increasing age. No such clear pattern could be observed with IgG-PGL and IgG-LAM.
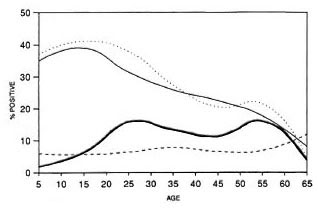
Fig. 2. Frequency of seropositives according to age. = MLPA;
= MLPA;  = IgM-PGL;
= IgM-PGL;  = IgG-PGL;
= IgG-PGL;  = IgG-LAM.
= IgG-LAM.
No significant relationship was found between the presence of a BCG scar and sero-positivity in any of the tests (results not shown).
Distribution of seropositivity among households. A total of 391 houses were occupied, giving an average occupancy rate of 4.9 persons. The average living space was 9.1 square meters per person. Scrum samples of persons belonging to 346 households were examined. No influence of the household size was found on the chance of being seropositive for any of the serological assays (Chi-squared test for trend, p > 0.05 for all tests). This is illustrated in Table 2 for MLPA. Two-hundred-scven households (59.8%) contained one or more MLPA-positive members.

In order to investigate whether the seropositivity rates were equally distributed among all households, the observed household seropositivity rate was compared with the expected household seropositivity rate for each household size. Nonrandom distribution of seropositivity was found for MLPA and IgM-PGL (Chi-squared = 60.8; p < 0.001 for MLPA; Chi-squared = 65.3, p < 0.001 for IgM-PGL), but not for the other two tests. Further analysis revealed that this finding could be attributed to eight households. In five households there was an excess of seropositive persons; these households comprised 21 seropositives among 24 persons tested. In three households seronegative persons were in excess; they consisted of 21 persons all of whom were negative. There was no difference between age or sex composition of the members of these two household groups, excluding confounding by age and sex (results not shown). The seropositivity rates among all other households conformed with a random distribution.
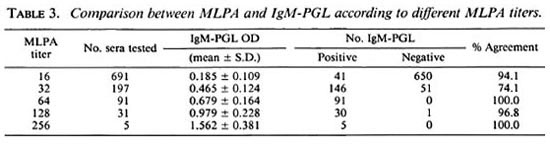
Comparison between serological tests. The results of the MLPA and the IgM-PGL to measure antibodies against the PGL-I antigen showed little differences. The MLPA test demonstrated antibodies in 32.0% of the population and the IgM-PGL in 30.8%. The overall agreement was 91.4% (Table 3). The agreement increased for higher titers for MLPA; whereas 51 of 197 samples with a titer of 1:32 (positive) were IgM-PGL-negative. There was only one serum with a titer higher than 1:32 (i.e., 1:128) which was negative in IgM-PGL.
Only weak correlations between the results could be established between all tests (r ranging from 0.14 to 0.24, with the exception of IgM-PGL versus MLPA [r = 0.82, p < 0.001]).
Relation serology and PCR. PCR results were obtained on 1228 nasal swab specimens. The overall positivity rate was 7.8% and did not show any relation to age or sex. Detailed results have been presented elsewhere C6). From 965 persons the results were obtained by both serology and PCR. In this group of persons the PCR positivity rate was 7.7%. No relationship could be detected between PCR positivity and seropositivity in the four serological assays, nor was there any correlation between PCR positivity and the height of the values obtained in the serological assays.
DISCUSSION
In this study we have applied four different serological assays and A PCR on specimens collected through A population-based survey in a high endemic area for leprosy. The results indicate widespread M. leprae infection in the population.
During the survey five new active leprosy patients were detected in each village, resulting in a similar registered prevalence of leprosy cases among the examined population of 10.7 and 9.9 per 1000 inhabitants, respectively. Therefore, results from the two villages were analyzed together. Since the studied population did not difTcr from the total population in its characteristics and, thus, was a representative sample thereof, it is justified to extrapolate the serological and PCR results of the whole population from both villages.
The seropositivity rates in leprosy patients in this study were similar to previous reports: high rates in multibacillary and low in paucibacillary patients (2).
Antibodies to PGL-I were demonstrated in 30.8% and 32% of the tested population by ELISA and the MLPA test. Assuming that the height of a serological response to PGL-I reflects the intensity of exposure to M. leprae (21), the large number of positives indicates a high level of exposure in this community. This is in agreement with our finding that the positivity rates in household contacts were similar to those of noncontacts, suggesting a uniform risk of exposure in this population. The results of other scrocpidemiological studies performed in South Sulawesi also implied a high risk ofcxposure in this population (20).
The scropositivity rates in MLPA and IgM-PGL in the population showed a similar age-related pattern as demonstrated before in other studies (11, 20): scropositivity was highest in the younger age groups and declined with increasing age. Noteworthy is the large number of seropositives in the age group 5-9 years, which is further evidence of widespread exposure. Our results showed A higher scropositivity rate among females than males, in conformity with what has been reported in a number of other studies (11, 21). The higher scropositivity rates among females did not reflect a higher case rate in our population; 4 out of 14 patients were female. Higher innate IgM levels among females compared to males is the most simple explanation for this phenomenon (17).
An interesting finding in this study was the observation that the scropositivity rates in the MLPA and IgM-PGL were not randomly distributed among all households. Five households were identified with an excess of seropositive members. In contrast, three other households had an excess of seronegative members. This observation is unlikely to be due to chance alone, and the composition of these households was similar with respect to age and sex. Clustering of scropositivity to PGL-I has been reported before among contacts of multibacillary index cases; the increased ability to shed bacilli by some index cases and the consequent exposure of household members to large numbers of bacilli has been put forward as one possible explanation for this clustering (9). Such a phenomenon could account for our finding of excessive scropositivity among certain households. A familial predisposition responsible for clustering, the other explanation, is in line with our observation of large households with only seronegatives. The persistence of this observed pattern of clustering needs to be established before it can be ascertained whether cither environmental or genetic factors or both are the underlying causes.
The MLPA and IgM-PGL showed an overall agreement of 91% and revealed a similar scropositivity profile in all aspects confirming reports of other studies (15). Since the MLPA test is much simpler to carry out, this test could be the test of choice, especially in less equipped laboratories. In addition, the MLPA has the advantage over the ELISA in that it is a standardized test, allowing a legitimate comparison between MLPA results of different scrocpidcmiological studies. The analysis of the results of the IgG-PGL and IgG-LAM did not reveal clear patterns, except for a male predominance of IgG-LAM scropositivity. The results of IgG-PGL and IgG-LAM did not correlate well with the results of the assays detecting IgM to PGL-I. It has been suggested that the LAM-based assays may detect additionally infected individuals compared to the PGL-I-based assays alone, assuming that certain individuals do react to M. leprae infection with predominantly an IgG response to LAM and not with an IgM response to PGL-I (8). Indeed, we also found more seropositive individuals when the assays were combined. Although the measurement of anti-LAM responses may be useful in the study of leprosy patients (8), the crossreactive nature of LAM complicates the interpretation of our results obtained in the healthy population.
In this study we have compared M. leprae -specific antibody responses and the presence of M. leprae in the nose as determined by PCR at a population level. No relationship between the two could be established. This finding may not be surprising since the presence of M. leprae in the nose does not necessarily imply infection. A transient state of carriership without colonization resulting in infection may have occurred. On the other hand, the discrepancy between PCR positivity and seropositivity also may be due to a time lag between the carriage of M. leprae in the nose and the onset of a serological response. Moreover, not every infection leads necessarily to a serological response, notably in those prone to get paucibacillary leprosy. Several followup studies have shown that new cases of leprosy also are found in the seronegative groups (1, 21). Whether measurement of nasal immunity may be of value in this respect has to be awaited (4). Since the nasal mucosa is probably the main port of entry, nasal immunity is likely to be an important defense against further dissemination of the bacilli.
It has been suggested that multibacillary cases are the main source of infection in a community. However, it is difficult to conceive how four multibacillary patients, two of whom had been treated for prolonged periods, would be responsible for an infection rate of at least 30% in this community. This high exposure rate suggests that there are important other sources of transmission. The finding that 7.8% of the population harbors M. leprae in the nose adds to that possibility.
Acknowledgment. We would like to express our gratitude to the Head of the Regional Office of the Ministry of Health, Republic of Indonesia in South Sulawesi, and to the leprosy officers in the Ministry of Health and in the Province of South Sulawesi for their cooperation. We are obliged to Dr. Peter Lever and Dr. Djumadi Achmed for their valuable help.
We are indebted to all of the people in the villages who voluntarily cooperated in this study.
The financial support by the Netherlands Leprosy Relief Association (NSL) and the European Communities Directorate General for Science, Research and Development (TS2-0275-NL) is greatly appreciated.
REFERENCES
1. BAGSHAWE, A. F., GARSIA, R. J., BAUMGART, K. and ASTBURY, L. IgM serum antibodies to phenolic glycolipid-I and clinical leprosy: two years' observation in a community with hypcrcndcmic leprosy. Int. J. Lepr. 58(1990)25-30.
2. CHO, S.-N., FUJIWARA, T., HUNTER, S. W. , REA, T. H. , GELBER. R. H. and BRENNAN, P. J. Use of an artificial antigen containing the 3,6-Di-Omcthyl-beta-D glucopyranosyl epitope for the scrodiagnosis of leprosy. J. Infect. Dis. 150(1984)311-322.
3. CHO, S.-N., KIM, S. H., CELLONA, R. V., CHAN, G. P., FAJARDO, T. T., WALSH, G. P. and KIM, J. D. Prevalence of IgM antibodies to phenolic glycolipid I among household contacts and controls in Korea and The Philippines. Lepr. Rev. 63(1992)12-20.
4. CREE, I. A., SHARPE, S., STURROCK, N. D. C, COCHRANE, I. H. , SMITH, W. C. and BECK, J. S. Mucosal immunity to mycobacteria in leprosy patients and their contacts. Lepr. Rev. 59(1988)309-316.
5. DAY, R., LEVER, P. and ASRI, M. Leprosy control in 7 districts of South Sulawesi, Indonesia, 1986-91. Lepr. Rev. 63(1992)247-254.
6. DE WIT, M. Y. L., DOUGLAS, J. T., MCFADDEN, J. and KLATSER, P. R. Polymerase chain reaction for detection of Mycobacterium leprae in nasal swab specimens. J. Clin. Microbiol. 31(1993)502-506.
7. DE WIT , M. Y. L., FABER, W. R., KRIEG, S. R., DOUGLAS, J. T, LUCAS, S. B., MONTREEWASUWAT, N., PATTYN, S. R., HUSSAIN, R., PONNIGHAUS, J. M., HARTSKEERL, R. A. and KLATSER, P. R. Application of a polymerase chain reaction for the detection of Mycobacterium leprae in skin tissues. J. Clin. Microbiol. 29(1991)906-910.
8. DHANDAYUTHAPANI, S., IZUMI, S., ANANDAN, D. and BHATIA, V. N. Specificity of IgG subclass antibodies in different clinical manifestations of leprosy. Clin. Exp. Immunol. 88(1992)253-257.
9. DOUGLAS, J. T., CELONA, R. V., ABALOS, R. M., MADARANG, M. G. and FAJARDO, T. Serological reactivity and early detection of leprosy among contacts of lepromatous patients in Cebu, The Philippines. Int. J. Lepr. 55(1987)718-721.
10. FINE, P. E. M. Reflections on the elimination of leprosy. Int. J. Lepr. 60(1992)71-80.
11. FINE, P. E., PONNIGHAUS, J. M., BURGESS, P., CLARKSON, J. A. and DRAPER, C. C. M. Scroepidemiological studies of leprosy in northern Malawi based on an enzyme-linked immuno-sorbent assay using synthetic glycoconjugatc antigen. Int. J. Lepr. 56(1988)243-254.
12. FUJIWARA T., ASPINALL, G. O., HUNTER, S. W. and BRENNAN, P . J. Chemical synthesis of the trisaccharide unit of the species-specific phenolic glycolipid of Mycobacterium leprae . Carbohydrate Res. 163(1987)41-52.
13. GILLIS, T. P. and WILLIAMS, D. L. Polymerase chain reaction and leprosy. Int. J. Lepr. 59(1991)311-316.
14. HARTSKEERL, R. A., DE WI T M. Y. L. and KLATSER, P. R. Polymerase chain reaction for the detection van Beers, et al.: Epidemiology of Leprosy Infection of Mycobacterium leprae . J. Gen. Microbiol. 135(1989)2357-2364.
15. IZUMI, S., FUJIWARA, T., IKEDA, M., NISHIMURA, Y., SUGIYAMA, K . and KAWATSU, K . Novel gelatin particle agglutination tests for serodiagnosis of leprosy in the field. J. Clin. Microbiol. 28(1990)525-529.
16. KLATSER, P. R. , VAN BEERS, S., MADJID, B., DAY, R. and DE WIT, M. Y. L. Detection of Mycobacterium leprae nasal carriage in leprosy endemic populations. J. Clin. Microbiol. 31(1993)2947- 2951.
17. MADDISON, S. E., STEWART, C. C, FARSHY, C. E. and REIMER, C. B. The relationship of race, sex and age to concentrations of serum immunoglobulins expressed in international units in healthy adults in the U.S.A. Bull. WHO 52(1975)179-185.
18. NORDEEN, S. K. , LOPEZ-BRAVO, L. and SUNDARE-SAN, T. K. Estimated number of leprosy cases in the world. Lepr. Rev. 63(1992)255-262.
19. RIDLEY, D . S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
20. SOEBONO, H. and KLATSER, P. R. A scroepidcmiological study of leprosy in high- and low-endemic Indonesian villages. Int. J. Lepr. 59(1991)416-425.
21. ULRICH, M., SMITH, P. G., SAMPSON, C, ZUNIGA, M., CENTENO, M., GARCIA, V. , MANRIQUE, X. , SALGADO, A. and CONVIT, J. IgM antibodies to native glycolipid-I in contacts of leprosy patients in Venezuela; epidemiological observations and a prospective study of the risk of leprosy. Int. J. Lepr. 59(1991)405-415.
22. WHO EXPERT COMMITTEE ON LEPROSY. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
1. M.Sc; Department of Microbiology, Hasanuddin University, Ujung Padang, South Sulawesi, Indonesia.
2. M.D., Department of Microbiology, Hasanuddin University, Ujung Padang, South Sulawesi, Indonesia.
3. Ph.D.; National Institute for Leprosy Research, Tokyo, Japan.
4. Technician, National Institute for Leprosy Research, Tokyo, Japan.
5. M.P.H., Leprosy Control, Directorate General of Communicable Disease Control and Environmental Health, Ministry of Health, Jakarta, Indonesia.
6. Ph.D., Department of Biomedical Research, Royal Tropical Institute, Mcibergdrcef 39, 1105 AZ Amsterdam, The Netherlands.
Reprint requests to Dr. Klatser.
Received for publication on 3 August 1993.
Accepted for publication on 26 October 1993.