- Volume 62 , Number 1
- Page: 99–107
Effect of Mycobacterium leprae in peripheral nerve trunk on the evolution of skin lesions
ABSTRACT
Comparative histological studies were made of a) 41 peripheral nerve lesions and the skin in the area of supply, and b) 12 peripheral nerve lesions and concurrent but unrelated skin lesions. In the first study, small, relatively early, histologically classifiable skin lesions were found in all cases, even though there were no clinical lesions. In every case the lesion was centered on a dermal nerve. In some cases disruption of the perineurium was associated with emergence of the lesion into the dermis and a small silent local reaction. It was concluded that there was a descending spread of the disease down the neural pathway to the dermis, although it was not necessarily associated with transport of bacilli.Although the first study showed a discrepancy in the classification between skin and nerve lesions in nearly 50% of the cases (as previously reported), the second study showed no discrepancies. It is suggested that discrepancies are relatively uncommon, and that those in the first study are exceptional. The probable explanation is that microreactions in the nerve trunks had caused a shift in classification, which was not yet reflected in the immature skin lesions. In the second study, the mature skin lesions had reached immunological equilibrium. Discrepancies in classification between skin and nerve lesions, as between concurrent skin lesions, are the result of reaction. Attention is drawn to the probable role of subliminal reactions in the evolution of infections.
RÉSUMÉ
Des études histologiques comparatives ont été réalisées de a) 41 lésions nerveuses périphériques ainsi que de la peau dans la région innervée, et b) 12 lésions nerveuse périphériques et des lésions cutanées simultanées mais sans rapport avec celles-ci. Dans la première étude, des petites lésions, relativement précoces et classifiables histologiquemcnt, ont été trouvées dans tous les cas, même s'il n'y avait pas de lésion clinique. Dans chaque cas, la lésion était centrée sur un nerf dermique. Dans certains cas, une interruption du périnèvre était associée à l'apparition de la lésion dans le derme, et à une petite réaction locale silencieuse. On en a conclu à une dissémination descendante de la maladie le long du trajet nerveux vers le derme, bien que cette dissémination n'était pas nécessairement associée au transport de bacilles.Bien que la première étude ait montré une discordance dans la classification entre les lésions nerveuses et cutanées dans près de 50% des cas (comme rapporté précédemment), la seconde étude n'a pa montré de discordances. L'hypothèse est émise que les discordances sont relativement peu fréquentes, et que celles de la première étude sont exceptionnelles. L'explication probable est que des micro-réactions survenues dans les troncs nerveux avaient causé un changement dans la classification, et ceci n'était pas encore reflété dans les lésions cutanées immatures. Dans la seconde étude, les lésions cutanées matures avaient atteint un équilibre immunologiquc. Des discordances de classification entre des lésions cutanées et nerveuses, comme entre des lésions cutanées simultanées, sont le résultant d'une réaction. L'attention est attirée sur le rôle probable de réactions subliminales dans l'évolution des infections.
RESUMEN
Se hicieron estudios histológicos comparativos de 41 lesiones de nervios periféricos y la piel de las áreas inervadas, y de 12 lesiones de nervios periféricos y la piel de lesiones dérmicas concurrentes pero no relacionadas. En todos los casos analizados en el primer estudio se encontraron lesiones dérmicas pequeñas, relativamente tempranas e histológicamente clasificables aún cuando no hubieron lesiones clínicas. En cada caso la lesión estuvo centrada en un nervio dérmico. En algunos casos la ruptura del perincurio estuvo asociada con la emergencia de la lesión hacia la dermis y con una pequeña reacción local silenciosa. Se concluyó que hubo una dispersión descendente de la enfermedad de la via neural a la dermis, aunque no necesariamente estuvo asociada con el transporte de bacilos.Aunque el primer estudio mostró discrepancias en la clasificación de las lesiones de la piel y de los nervios, en casi el 50% de los casos (como se reportó previamente), en el segundo estudio no hubieron discrepancias. Se sugiere que las discrepancias son relativamente raras y que aquellas del primer estudio son excepcionales. Probablemente las micro-reacciones en los troneos nerviosos, sin reflejo aún en las lesiones inmaduras de la piel, fueron la causa del cambio en la clasificación. En el segundo estudio, las lesiones maduras de la piel ya habían alcanzado su equilibrio inmunológico. Las discrepancias en la clasificación entre las lesiones de la piel y de los nervios, así como las discrepancias entre las lesiones concurrentes de la piel, son probablemente el resultado de reacciones. Se hace incapié sobre el probable papel de las reacciones subliminales en la evolución de las lesiones de la lepra.
Peripheral nerves in leprosy constitute a reservoir of bacilli that is relatively inaccessible to investigation, which is thought to be secluded from immunological detection, and to represent a potential hazard for the spread of the infection, the induction of reactions, and perhaps after treatment for the precipitation of relapse. What is questioned is not the hazard but the manner of its realization. As to the spread of infection via neural pathways, the ascending route from nerve filaments in skin or mucous membrane to nerve trunk is well established (5). Whether descending infections in the reverse order are a significant factor in pathogenesis, and under what circumstances, is not clear. Against it is the persistence in some cases of the pure neural state, with lack of dermal involvement even when bacillary loads are high (6). After treatment, a proportion develop skin lesions (20) but Mycobacterium leprae may still remain confined to nerve twigs for periods of years (13). On the other hand, without some movement from nerve to skin the role of the neural reservoir is unexplained. Diffusion of infection through the perineurium, in either direction, is a possibility (2). On the hypothesis supported by our previous studies (15, 16), the emergence of organisms from dermal nerves probably would coincide with their immunological exposure, and so be significant for the induction of reactions.
A series of concurrent nerve and skin biopsies appeared to be particularly appropriate for a study of the emergence of leprosy bacilli from neural pathways and the possible evolution of new skin lesions, because the skin biopsies were taken from the relatively healthy areas of skin supplied by the peripheral nerves. We have already made use of this series of biopsies in two previous studies (15, 16). Then our attention was directed mainly to the peripheral nerve. Here we are concerned more with the dermal component, although still in relation to the neural involvement. For comparison with these relatively early and immature skin lesions we looked also at the nerve-dermal relationship in some more-advanced skin lesions and in type 1 (delayed hypersensitivity) reactions.
MATERIAL AND METHODS
Patients with leprosy. There were three groups of leprosy patients: 1) 41 untreated patients with clinically enlarged and painful peripheral nerves received concurrent biopsies of the affected nerve and the area of skin supplied by it, irrespective of the presence or absence of clinical involvement of the skin (in most cases there was no apparent involvement). None of the patients was in reaction clinically. 2) 12 untreated patients with clinically enlarged painful nerves, who had skin lesions in addition, received concurrent biopsies of both nerve lesion and skin lesion, irrespective of the relationship between the sites (in most cases there was no relationship). None of the patients was in reaction clinically. Post-treatment biopsies taken after 1 to 3 years of multidrug therapy (MDT) were also examined. 3) 15 patients in clinical reaction (type 1) received biopsy of the reacting skin lesion. Nerves were not biopsied. In no case was there any clinical evidence of involvement of the peripheral nerve that supplied the skin area. Most of these patients in this group were under treatment; pretreatment and posttreatment skin biopsies were available for comparison.
The biopsies of the first group were taken by the late Dr. J. C. Pedley from patients in Nepal. Patients of the second group were under our care at the Hospital for Tropical Diseases, London, and those of the third group were at the same Hospital or in the Sungei Buloh Leprosarium, Malaysia.
Processing of biopsies. The biopsy specimens of group 1 had been fixed in FMA (10 ml formalin, 2 g mercuric chloride, 3 ml acetic acid in 100 ml distilled water) for 12 hr according to Harman, and stored in 70% alcohol. The biopsies of the other two groups were fixed in FMA for 1 ½ -3 hr. Sections were stained by hematoxylin-cosin and by the Wade-Fite method for acid-fast bacilli (AFB).
Immunoperoxidase. All biopsies of groups 1 and 3 were stained by the peroxidase-antiperoxidase (PAP) technique, using anti-BCG antibody to detect M. leprae antigen, as previously (16). After washing in Tris-buffered saline (0.05 M, pH 7.4), the following reagents were applied in sequence: normal swine serum 1/10 for 30 min, Tris saline wash, swine anti-rabbit antibody 1/20 for 30 min, Tris saline wash, and finally the PAP reagent 1/50 for 30 min. This was done in a moist chamber at room temperature. The reaction product was developed with DAB (0.05% diaminobenzidine tetrahydrochloride; Sigma Chemical Co., St. Louis, Missouri, U.S.A.) in 0.01% hydrogen peroxide diluted in Tris saline, 2 min. Sections were counterstained with Mayer's hemalum.
Selected paired biopsies of nerve and skin (group 1) and skin only (group 3) were stained also with S-100 IgG antiserum at 1/200 to identify Schwann cells by the PAP method. Relevant controls using normal rabbit scrum in place of the primary antibody were used throughout.
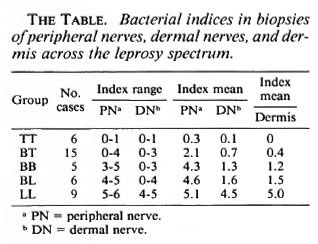
Method of examination. The 41 dual biopsies of group 1 were examined to note the proportion of granuloma, and/or AFB-containing Schwann cells, in peripheral nerve trunk, epineurium and dermal nerves. The results were recorded as: + + + = heavy infiltration, + + = moderate or quiescent, + = very little. The nature of any lesion in the dermal nerves was noted. Biopsies of group 2 were examined similarly. The density of AFB, determined on a logarithmic scale from 0 to 6+ in each case, was also compared to the density in the dermis. Finally, the results in these two groups were compared with those of the reacting lesions in group 3.
Classification of skin biopsies. Skin biopsies were classified by the Ridley-Jopling method. Nerve trunk lesions, which do not exactly parallel those of skin, were classified by a modification which emphasizes granuloma cell type (5). The 41 nerve lesions of group 1 comprised 6 TT, 15 BT, 5 BB, 6 BL and 9 LL; 4 of the BT cases showed features of histological reaction (BTR). The 12 cases of group 2 comprised 6 BT, 4 BL and 2 LLs (1 later upgraded to BL). Among the 15 reactions of group 3, 7 appeared to be upgrading (3 BT → TT, 4 BL → BT), 3 downgrading (BT → BL), and 5 were static with no change of classification (1 BT, 4BL).
RESULTS
The dual peripheral nerve and skin biopsies of group 1 provided a comparison of concurrent states of the disease in peripheral nerve, dermal nerve and the surrounding dermis.
Peripheral nerves
Peripheral nerves demonstrated the usual aspects of the pathology of leprosy across its whole spectrum. An epithelioid cell or macrophage granuloma was present in many cases (TT-BL), replacing part of the nerve fascicles; in BT it was most variable in extent. In LL, Schwann cells heavily laden with AFB largely took the place of granulomas. The same type of granuloma affected the epineurium. As the number of AFB increased from TT to LL, there was a corresponding decrease in the inflammatory cells in the epineurium; reduplication of its connective tissue layers was not affected. Microrcactions, involving parts of a fascicle or whole fascicles, were a feature of a majority of these peripheral nerve lesions, despite the absence of clinically apparent reaction. They were found throughout the spectrum, except in LL, and were characterized by edema, necrosis and other features of type 1 reactions, with diminution of bacterial antigen.
Dermal nerves
Dermal nerve bundles were identifiable in all of the 41 skin biopsies. Over most of the spectrum (all the TT-BL cases), the lesion was more or less confined to the dermal nerves, with only slight extension to the dermis. Only in 9 LL lesions were loose, early lepromas seen in close association with the perineurium of relatively normal nerve bundles. Although the lesions were sufficiently advanced to be classifiable in 40 of the 41 cases, they were histologically immature as well as clinically inapparent. The histology could be graded into three patterns according to the response within the neural component, although with some overlap between them. The three responses were: a) early neural, b) neural granuloma,and c) disrupted perineurium.
a) Early neural. A mantle of mononuclear cells surrounded the intact perineurium of small-to-medium sized nerve bundles (Fig. 1). The neural structure also was fairly intact despite the possible presence of some bacilli. A similar cellular mantle affected the small blood vessels in some of the skins in BB, BL and LL cases.
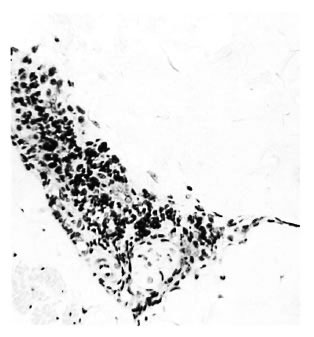
Fig. 1. Borderline tuberculoid "a" response showing intact nerve with perineurial lymphocytes and mononuclears; group 1 ( x 250).
b) Neural granuloma. Within an intact perineurium the nerve bundle was occupied and mostly replaced by a granuloma which was often similar to that infiltrating the peripheral nerve (Fig. 2).
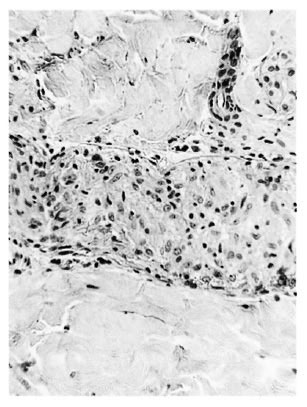
Fig. 2. Borderline tuberculoid "b" response showing intraneural epithelioid cell granuloma and some surviving Schwann cells containing 2+ AFB within an intact perineurium; group 1 ( x 250).
c) Disrupted perineurium. The dermal perineurium was disrupted with some cellular necrosis and release of endoneurialgranuloma (Fig. 3), also Schwann cells, macrophages and perhaps giant cells. Sometimes there was dispersal of scanty AFB. Epithelioid cells were usually present. For the most part the lesion was strictly localized around the disrupted nerve bundle. However, in some cases there might be local edema and severe connective tissue inflammation, signifying an incipient subclinical reaction, type 1 (Fig. 4).
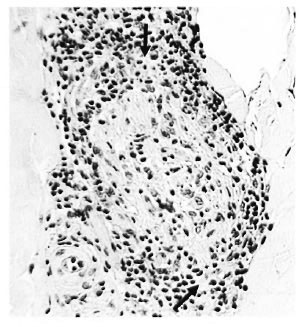
Fig. 3. Borderline lepromatous "c" response showing disrupted perineurium with localized breakout of macrophages (↑) containing 3+ AFB; group 1 ( x 250).
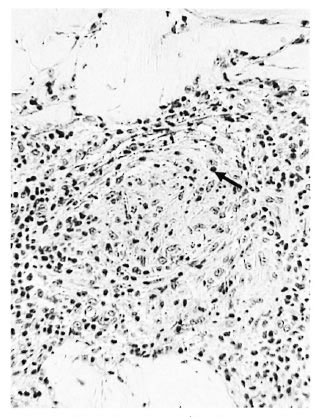
Fig. 4. Borderline tuberculoid "c" response showing a more advanced stage with disrupted perineurium, necrosis and pyknosis (↑), and epithelioid cells associated with it. Incipient reaction at the site of dermal nerve and dermis; group 1 ( x 250).
These three histological responses in dermal nerve appeared to evolve in progression from "a" to "c." This was a general impression from the large group 1 series of cases, which was confirmed by serial biopsyin 4 ofthe 12 cases of group 2. The responses were not significantly associated with the classification of the peripheral nerves. The early neural response was associated withperipheral nerve classifications of TT in 1, BT in 3, BB in 2, and BL in 5 cases. Dermal nerve granuloma was associated with 3 TT and 8 BT or BB peripheral nerves, the granuloma being early with many lymphocytes in the BB and BT cases, fully developed with epithelioid cells in TT. Disrupted perineurium was associated with 2 TT, 6 BT and 1 BB peripheral nerves. These responses were not related to either the extent or activity of the corresponding peripheral nerve lesion or to the number or form of the bacilli within it. However, a severely disrupted perineurium usually coincided with epincurial inflammation.
Acid-fast bacilli. Larger numbers of AFB in peripheral nerve were associated with infection of Schwann cells and granuloma, while the dermal nerves and dermis as a rule showed fewer bacilli (The Table). Solidstaining organisms were usually seen in Schwann cells throughout the neural pathway, granular forms lodged in granuloma cells.
Evolution of dermal lesions
The skin lesions of group 2, unlike those of group 1, were clinically apparent. Histologically they were more advanced. They were unrelated to the nerve lesions biopsied, but the peripheral nerve lesions of the two groups were in all respects similar and comparable. The group 2 lesions therefore were taken to represent a later phase in the evolution of the neural-dermal relationship. In group 2 the more advanced stage of the skin lesions was denoted by the same type of granuloma affecting equally the dermis and neurovascular bundles. There was reactive proliferation of the perineurium, and lymphocytic or granulomatous infiltration of the dermal adnexa (Fig. 5). However, the three types of dermal nerve responses were still identifiable, some at a more advanced stage. The early neural response was now represented by a compact perincurial cellular mantle, which equated with BL. More cases fell into the neural granuloma group, which showed thickened perineuria.

Fig. 5. Borderline tuberculoid "b" response showing perineurial layering (↑), an intraneural granuloma, and a large established granulomatous lesion outside the perineurium; group 2 (x 250).
The dermal component of the lesion was larger in relation to the neural involvement, and the relationship less distinct. The three types of responses ceased to be identifiable after treatment by MDT for 2 to 3 years, dermal nerves then showing no more than a mild chronic neuritis.
Discrepancy in classification. There was one significant difference between groups 1 and 2. In group 1 there was a discrepancy between the histological classifications of peripheral nerve and skin in nearly half of the cases (18 out of 40), as previously reported (15). In the 12 cases of group 2 there were no discrepancies between the classifications of skin and nerve lesions.
Neural involvement and dermal reaction
Microreactions, as seen in the peripheral nerves, were not detected in any of the dermal nerves of any of the groups. Reactions only became incipient in the dermis as the disease process broke out from the nerve bundles (the perineurial disruption response). At this stage the reaction (type 1) was already situated mainly in the dermis (Figs. 3 and 4).
In the 15 cases of advanced clinically apparent reaction (group 3), dermal nerves were identifiable in the pretreatment and prereaction biopsies, showing the same sorts of responses as in group 2. But at the peak of the reaction the dermal nerves had been largely destroyed (Fig. 6a) and their possible involvement in the initiation of the reaction could not be elucidated. Even after staining for Schwann cells by S-100 antibody, only damaged nerves, or none at all, were seen. On subsidence of the reaction the few detectable nerves showed a mild chronic neuritis (Fig. 6b).
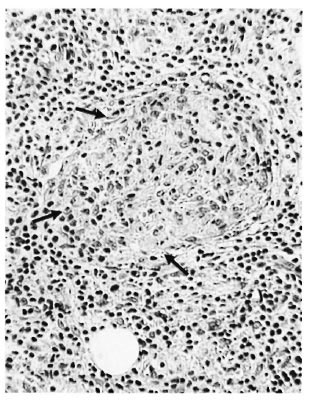
Fig. 6a. Upgrading reaction, BL→BT, showing enlarged nerve with perineurial disruption (↑) and inflammatory cells all around; group 3 ( x 250).
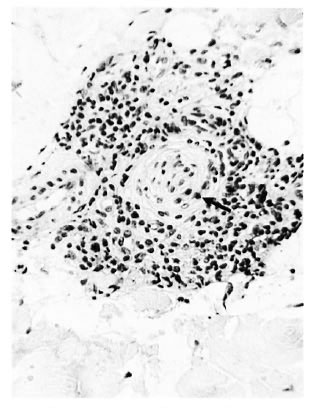
Fig 6b. Same case as Fig. 6a showing subsidence of the reaction and a relatively normal nerve with perineurial layering (↑) and lymphocytes ( x 250).
DISCUSSION
The first observation of this study was that peripheral nerve lesions were associated in all cases with histological involvement in the area of skin supplied by the nerve, even when no skin lesion was clinically apparent (group 1). Other authors had found no skin lesion in small numbers of cases in parallel studies ( 7, 10, 11 ) . Furthermore, our skin lesions, although smaller and histologically less mature than those of the related peripheral nerve, were sufficiently established to be classifiable within the spectrum. Studies of early skin lesions inleprosy usually show a progression from focal lymphocytic infiltration to granuloma, affecting at first either subepidermal zone, neurovascular bundles or skin adnexa (2, 4, 12, 19). Our results correlated well with these reports, except that the lesions were entirely perineural. In the earlier cases they did not involve the extraneous dermis, but in the more advanced lesions, there was breakdown of the perineurium of a dermal nerve, with spread of the lesion to the surrounding dermis.
The incipient skin lesions clearly had originated by spread from the nerves, which does not necessarily indicate that leprosy bacilli had progressed distally down the nerve trunk. It may be that the whole neural pathway had become affected at the same time, perhaps by hematogenous dissemination, and that the peripheral nerve trunk was preferentially involved more than the dermal nerves. The lesion could have spread as the immune response evolved in recognition of the increasing amounts of bacterial antigen, aided by breakdown of neural structure through microrcactions (16). Thus, infections probably do "descend" down neural pathways, whether or not motion is involved. Chandi and Chacko (2) thought that their finding of more bacilli in the epineurium and perineurium (which we confirmed in a few cases) might suggest either a hemic spread via epincural vessels or a filtration process across the damaged membrane. It seems that there are two opposing perineurial responses, a proliferative thickening e 2-17 ) which would inhibit passage of bacilli, and breakdown of the perineurium which would facilitate the spread of infection. It is not yet clear whether the latter is the cause or the result of reaction at the site.
Our second main observation was that despite a discrepancy between the histological classification of the neural and dermal lesions in nearly 50% of our group 1 cases, as previously reported (15), there were no such discrepancies in classification in the 12 cases of group 2. The result was a surprise, the more so since the group 2 skin and nerve lesions were unrelated to each other. A review of the literature suggests that such discrepancies might be quite few. Many studies on parallel nerve and skin biopsies foundno discrepancies (3,8, 12). Srinivasan, et al. (18) are careful to describe their findings asdiscrepancies of histopathology. They tabulate discrepancies in classification, but themajority are not more than the difference between classifiable and unclassifiable lesions; true differences amounted to 6 out of 36. Similar comments would apply to someother reports (7, 9, 11). It is likely, therefore, that discrepancies in the classification of skin and nerve lesions are unusual, and it is our group 1 cases that are the exception. The explanation may lie in the discrepancy between the immaturity of the skin lesions compared with the maturity of the nerve lesions.
The classification of multiple concurrent skin lesions is generally uniform (again provided the lesions are classifiable). Discrepancies occur very largely during, or as a result of, upgrading type 1 reactions (14). We suggest that a similar explanation is the key to the discrepancies between nerve and skin lesions, namely, the microreactions in peripheral nerves, which we have previously reported in more detail (16). Clinically silent, they are a regular feature of peripheral nerve involvement. They show the features of hypersensitivity reactions, which are often associated with upgrading or downgrading, and can be seen to be associated with local alterations in the classification of nerve lesions. In regard to the skin lesions, the immature lesions of group 1 were insufficiently evolved to have undergone reaction; "early" skin lesions are slow to evolve (1). In consequence there are discrepancies of classification. The more advanced lesions of group 2, whether or not they originated from the dermal nerves, would have had time enough for reactions, silent or otherwise, to reestablish the immunological equilibrium; there were no discrepancies. The implication is that discrepancies in classification between nerve and skin lesions are the result of reactions, and that they reflect the immunological imbalance associated with neural protection.
We have attributed microreactions in peripheral nerve to the destruction of Schwann cells and the immunological exposure of previously occluded antigen. In dermal nerves there were no microrcactions because any such event would result in the rupture of the perineurium and the release of exposed antigen into the dermis. This was observed in the "c" response, and the small, local skin reaction that ensued was again clinically silent. It was histologically comparable to the advanced reactions of group 3, but at that late stage most of the dermal nerves had been destroyed without a trace and neural relationships could not be studied. Subliminal reactions are perhaps the normal mode of attaining immunological equilibrium in leprosy, becoming overt only when a reaction is severe enough to become self-perpetuating or to engage the entire mass of bacterial antigen. The pathogenic role of subliminal reactions, either in nerve trunk or skin lesion, has received little attention. Subliminal reactions could well be the explanation for the upgrading or downgrading that occurs, sometimes in association with neuritis, in the absence of overt reaction.
Acknowledgment. We gratefully acknowledge the valuable assistance of Dr. S. B. Lucas in supplying sections of the group 2 cases. The work was supported by the Spécial Trustées of University Collège Hospital, for which MJR is grateful.
REFERENCES
1. BUNGELER, W. Die pathologische Anatomic der Lepra. Virchow's Arch. (A) 310(1943)493-565.
2. CHANDI, S. M. and CHACKO, C. J. G. An ultrastructural study of dermal nerves in early human leprosy. Int. J. Lepr. 55(1987)570-575.
3. HAIMANOT, R. T., MSHANA, R. W., MCDOUGALL, A. C. and ANDERSON, J. G. Sural nerve biopsy in leprosy patients after varying periods of treatment. Histopathological and bacteriological findings on light microscopy. Int. J. Lepr. 52(1984)163-170.
4. IYER, C. G. S. Predilection of M. leprae for nerves; neurohistopathological observations. Int. J. Lepr. 33(1965)634-645.
5. JOB, C. K. Nerve damage in leprosy. Int. J. Lepr. 57(1989)532-540.
6. KAUR, G., GIRDHAR, B. K., GIRDHAR, A., MALAVIYA, G. N., MUKHERJEE, A., SENGUPTA, V. and DESIKAN, K. V. A clinical, immunological and histological study of neuritic leprosy patients. Int. J. Lepr. 59(1991)385-392.
7. KAUR, S., SHARMA, V. K, BASAK, P., KAUR, I. and RADOTRA, B. D. Concurrent skin and nerve histology in leprosy and its role in classification of leprosy. Lepr. Rev. 64(1993)110-116.
8. Liu, T.-C. and Qui, J.-S. Pathological findings on peripheral nerves, lymph nodes and visceral organs of leprosy. Int. J. Lepr. 52(1984)377-383.
9. MUKHERJEE, A. and MISRA, R. S. Comparative histology of skin and nerve granulomas in leprosy patients. Lepr. Rev. 59(1988)177-180.
10. NEGESSE, Y., BEIMNET, K, MIKO, T., WONDIMUS, A. and BERHAN, T. Y. In leprosy the presence of mycobacteria in the nerve is an essential factor in the cycle and spectrum of M. leprae infection. Lepr. Rev. 64(1993) 104-109.
11. NILSEN, R., MENGISTU, G. and REDDY, B. B. The role of nerve biopsies in the diagnosis and management of leprosy. Lepr. Rev. 60(1989)28-32.
12. PEARSON, J. M. H. and WEDDELL, A. G. M. Perineuria! changes in untreated leprosy. Lepr. Rev. 46(1975)52-67.
13. PEREIRA, J. H., PALANDE, D. D. and GSCHMEISS-NER, S. E. Mycobacteria in nerve trunks of longterm treated leprosy patients. Lepr. Rev. 62(1991)134-142.
14. RIDLEY, D. S. Pathogenesis of Leprosy and Related Diseases. London: Butterworth, 1988, pp. 165-168.
15. RIDLEY, D. S. and RIDLEY, M. J. Classification of nerves is modified by the delayed recognition of M. leprae . Int. J. Lepr. 54(1986)596-606.
16. RIDLEY, M. J., WATERS, M. F. R. and RIDLEY, D. S. Events surrounding the recognition of M. leprae in nerves. Int. J. Lepr. 55(1987)99-108.
17. SHETTY, V. S., MEHTA, L. N., IRANI, P. F. and ANTIA, N. H. Study of the evolution of nerve damage in leprosy. Part 1. Lesions of the index branch of the radial cutaneous nerve in early leprosy. Lepr. India 52(1980)5-18.
18. SRINIVASAN, H., RAO, K. S. and IYER, C. G. S. Discrepancy in the histopathological features of leprosy lesions in the skin and peripheral nerves. Lepr. India 54(1982)275-286.
19. TAKASHI, D., ANDRADE, H. F., JR., WAKAMATSU, A., SIQUEIRA, S. and DE BRJTO, T. Indeterminate leprosy: histopathologic and histochemical predictive parameters involved in its possible change to paucibacillary or multibacillary leprosy. Int. J. Lepr. 59(1991)12-19.
20. WATERS, M. F. R., RIDLEY, D. S. and RIDLEY, M. J. Clinical problems in the initiation and assessment of multidrug therapy. Lepr. Rev. 57Suppl.3(1986)92-100.
1. Ph.D.; Hospital for Tropical Diseases, 4 St. Pancras Way, London NW1 OPE, U.K.
2. M.B., F.R.C.P., F.R.C.Path., Hospital for Tropical Diseases, 4 St. Pancras Way, London NW1 OPE, U.K.
3. M.D., F.R.C.Path., 32 Basildon Court, 28 Devonshire Street, London NW1 1RH, U.K.
Received for publication on 6 September 1993.
Accepted for publication on 19 November 1993.