- Volume 62 , Number 1
- Page: 48–54
Denatured muscle grafts for nerve repair in an experimental model of nerve damage in leprosy. 1. A functional and morphometric study
ABSTRACT
The effectiveness of denatured autologous muscle grafts for nerve repair in an experimental model of leprosy was assessed. Nerve damage resembling that caused by Mycobacterium leprae in humans was induced by the injection of cobalt-irradiated M. leprae into the tibial nerve of guinea pigs. At the time of maximum functional loss, caused by the formation of a granuloma within the nerve, the area of damage was excised and a denatured autologous muscle graft was used to repair the nerve. Assessment of nerve regeneration through the graft was made using clinical, electrophysiological and microscopic morphometric analysis at intervals up to 20 weeks. The results were compared with regeneration after grafting of a normal nerve. Clinically, some motor and sensory recovery occurred in all of the graft recipients in the normal nerve by 8 weeks, and by 11 weeks in the recipients of grafts in the granulomatous nerve. Full sensory recovery occurred in all but one animal by 20 weeks. Motor function recovered to near normal levels at 14 weeks after repair of the normal nerve but, at 20 weeks, there was variation in motor recovery after repair of the granulomatous nerve. Electrophysiology showed increased conduction velocity of the nerve fibers at each timepoint. The conduction velocity at 8 weeks after grafting of the normal nerve was similar to that at 12 weeks after grafting of the granulomatous nerve. Morphometry showed an increasing number of myelinated fibers repopulating the distal nerve up to 20 weeks. Myelin fiber numbers, at this time, were one third of normal after repair of the granulomatous nerve and two thirds after repair of the normal nerve. This study demonstrates that denaturcd autologous muscle grafls enable tlie regeneration and functional rccovery of nerves dcspitc their bcing damaged by mycobacteria-induced granulomas, but the damage causes some delay.RÉSUMÉ
On a evalué refficacité de greffes dénaturécs de muscles autologues pour la réparation nerveuse dans un modele experimental de la lepre. Des lésions nerveuses ressemblant à celles causées par le Mycobacterium leprae chez l'homme ont été produites par l'injection de M. leprae irradiés au cobalt dans le nerf tibial de cobayes. Au moment de la perte maximale de fonction, causée par la formation d'un granulome à l'intérieur du nerf, la zone endommagée a été excisée et un greffon dénaturé de muscle antologue a été utilisé pour réparer le nerf. Une évaluation de la régénération nerveuse à travers le greffon a été faite sur base d'analyses morphométriques cliniques, électrophysiologiques et microscopiques à intervalles, jusqu'à la vingtième semaine. Les résultats ont été comparés avec la régénération après la greffe sur un nerf normal. Cliniquement, une certaine récupération motrice et sensorielle est survenue chez tous les receveurs de greffe dans le nerf normal à la huitième semaine, et à la onzième semaine chez les receveurs de greffe dans le nerf granulomateux. Une récupération sensorielle complète survint chez tous les animaux à l'exception d'un seul à la vingtième semaine. La fonction motrice était revenue à des niveaux pratiquement normaux 14 semaines après réparation du nerf normal, mais à 20 semaines, il y avait des variations dans la récupération motrice après intervention sur nerf granulomatcux. L'électrophysiologie a montré une augmentation de la vitesse de conduction des fibres nerveuses à chaque moment. La vitesse de conduction 8 semaines après la greffe du nerf normal était semblable à celle observée 12 semaines après la greffe sur le nerf granulomatcux. La morphométrie a montré une augmentation des libres myélinisées recolonisant le nerf distal jusqu'à la vingtième semaine. A ce moment, le nombre des fibres de myéline était le tiers de la normale après réparation du nerf granulomatcux, et deux-tiers après réparation du nerf normal. Cette étude démontre que les greffons dénaturés de muscle autologue peuvent produire la régénération et la récupération fonctionnelle des nerfs malgré qu'ils aient été endommagés par des granulomes provoqués par des mycobactérics, mais le dommage cause un certain retard.RESUMEN
Usando un modelo experimental de la lepra se estableció la efectividad de los injertos autólogos de músculo desnaturalizado para promover la reparación de nervios. La inyección de Mycobacterium leprae irradiado con cobalto en el nervio tibial del cobayo, condujo al desarrollo de una lesión nerviosa similar a la causada por M. leprae en el humano. En el tiempo de máxima pérdida funcional (causada por la formación de un granuloma dentro del nervio) se extirpó el área dañada y se substituyó por un implante autólogo de músculo desnaturalizado con objeto de reparar el nervio. El grado de regeneración nerviosa se estableció por criterios clínicos, electrolísiológicos y morfométrico-microscópicos realizados a intervalos hasta cubrir 20 semanas. Los resultados se compararon con la regeneración observada cuando el injerto de músculo desnaturalizado se hizo en un nervio normal. Mientras que todos los recipientes del injerto en el nervio normal mostraron un cierto grado de recuperación motora y sensorial hacia la octava semana, los injertados en el nervio granulomatoso mostraron un grado comparable de recuperación sólo hasta la onecava semana. Hacia las 20 semanas todos los animales, menos uno, mostraron completa recuperación sensitiva. La función motora se recuperó a niveles casi normales 14 semanas después de la reparación del nervio normal, en cambio, los animales en los que se hizo la reparación del nervio granulomatoso mostraron variaciones en la recuperación motora aun a las 20 semanas. La electrofisiología mostró una incrementada velocidad de conducción délas fibras nerviosas en cada tiempo estudiado. La velocidad de conducción a las 8 semanas en el nervio normal injertado fue similar a la velocidad de conducción a las 12 semanas del injerto en el nervio granulomatoso. La morfometria mostró que el número de libras mielinizadas en el nervio distal aumentó progresivamente hasta las 20 semanas. Los números de libras de miclina, en este tiempo, fueron un tercio de lo normal después de la reparación del nervio granulomatoso y de dos tercios después de la reparación del nervio normal. Este estudio demuestra que los injertos de músculo autólogo desnaturalizados permiten la regeneración y la recuperación funcional de los nervios, no obstante que estos hayan sido dañados por los granulomas inducidos por las micobaclerias, aunque el daño es causa de cierto retraso en la recuperación.Leprosy is a chronic infectious disease of man, with a worldwide prevalence of 5.5 million (10). The causative organism, Mycobacterium leprae , has a predilection for nerves, and leprosy is probably the most common cause of peripheral neuropathy in endemic countries. Despite drug treatment, over 20% of leprosy patients develop localized peripheral nerve damage due to intraneural granulomas (1,5).
Some patients can benefit from steroid therapy and surgical decompression of the nerve but a significant number still go on to develop irreversible nerve damage (1, 3). Loss of the protective sensation in the hands and feet leads to ulceration, mutilations and deformities. Although tendon-transfer operations can alleviate some of the effects of motor paralysis, there currently is no standard procedure for correcting sensory deficit, which requires nerve fiber regeneration (2). Excision of the granulomatous lesion and replacement with a nerve autograft has been tried, largely unsuccessfully mainly due to lack of disease-free, donor material of sufficient size and length (16).
The use of autologous muscle grafts for nerve repair eliminates the problems of compatibility, availability and length of the graft material. This technique has been used for the repair of transected nerves in experimental animals (8) and in man (11, 12). Preliminary work has been carried out on nerve repair in an experimental model of leprosy. The injection of M. bovis BCG or cobalt-irradiated M. leprae organisms into the sciatic nerve of guinea pigs induced intraneural granulomas and nerve damage with some features similar to that found in leprosy patients. In the M. leprae model, infiltration of macrophages, a thickened perineurium, fibrosis and axonal damage were seen (4). This model was modified by injecting the M. leprae organisms into the largest tibial fascicle of the sciatic nerve to give a localized lesion confined to one nerve. The granulomatous area of the nerve was excised and replaced with a denatured autologous muscle graft. Clinical and histological assessment at 50 and 150 days after muscle grafting showed nerve regeneration through the graft to repopulate the distal nerve and some motor and sensory recovery (13).
The damage to the distal nerve in leprosy neuritis, as opposed to simple transection found in most trauma cases, may affect nerve regeneration and functional recovery following muscle grafting. The present study reports on the rate of nerve regeneration and functional recovery after muscle grafting of the M. leprae -damaged nerve in guinea pigs. A comparison is made with regeneration following muscle grafting of a normal nerve. Morphometric analysis on semithin sections was used to measure the number, size and myelin sheath thickness of the regenerated myelinated axons in the tibial nerve distal to the graft. Conduction velocities of the regenerated axons were assessed electrophysiologically.
MATERIALS AND METHODS
Animals. Outbred, Hartley-strain, female guinea pigs, weighing 250-300 g, were obtained from David Hall (Stafford, U.K.). Animals were fed on RGP pelleted diet ad lib. , supplemented with cabbage.
Mycobacteria. Cobalt-irradiated (2.5 Mrad), armadillo-derived M. leprae was donated by Dr. R. J. W. Rces (National Institute for Medical Research, London, U.K.) through the World Health Organization IMMLEP program.
Induction of intraneural granuloma. The guinea pigs were anesthetized with 0.5 ml/ kg "Hypnorm" (0.315 mg/ml fentanyl citrate and 10 mg/ml fluanisone; Janssen Pharmaceuticals, Oxford, U.K.) and 0.25 mg/kg midazolam ("Hypnovel"; Roche, Welwyn Garden City, U.K.) given intramuscularly in the forelimb. The right sciatic nerve was exposed aseptically by dividing the biceps femoris muscle and the largest fascicle of the tibial nerve isolated. This nerve innervates parts of the lower leg including the flexor digitorum longus (FDL) muscle and the center of the sole of the foot. Using a microsyringe and a 33-gauge needle, 10 µ l of mycobacterial suspension containing 108 M. leprae organisms were slowly injected intraneurally. The muscle and skin were closed using 4/0 Coated Vicryl (Ethicon, U.K.). The animals were monitored until full recovery from the anesthetic. They were left for 5 weeks, the time of maximal nerve damage.
Muscle graft. The tibial nerve was isolated as before, and the area of granulomatous damage excised to leave a gap of at least 1 cm. A portion of the vastus lateralis muscle where the fibers lie in parallel bundles was excised. The muscle was denatured by freezing completely in an instant freezing aerosol (Lipfreeze; L.I.P. Ltd., Shipley, U.K.) and then thawing in sterile distilled water. The muscle was trimmed to fit the gap. The ends of the tailored graft were sutured to the perineurium of the nerve with 4 to 6 10/0 prolcne sutures (Ethicon) and the wound closed. Animals were killed 8, 12, 16 or 20 weeks after grafting with at least six animals per group.
A group of 12 normal animals of the same age, at grafting, as those with a granuloma in the nerve were given a muscle graft after excision of a 1-cm portion of the tibial fascicle of the sciatic nerve. Six were killed at 8 weeks after grafting; the others were killed at 20 weeks.
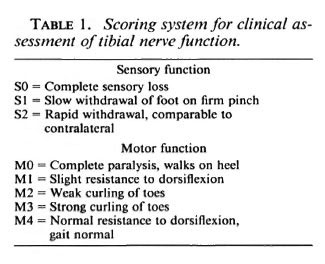
Clinical assessment. Motor and sensory functioning of the tibial nerve were assessed twice weekly by the scoring system shown in Table 1 (13). Sensory recovery was measured by the response to pinching the center of the sole of the foot in the area innervated only by the tibial nerve. Detailed observations were made of the clinical damage and the pattern of recovery after transection of the sciatic, femoral, tibial and peroneal nerves which showed no evidence of double innervation (13). The strength of toe curling and resistance to dorsiflexion (functions of the FDL) were used to assess motor function. Normal responses were checked using the contralateral (left) foot as a control.
Electrophysiological assessment. At 8, 12, 16 and 20 weeks after grafting of the granulomatous nerve and 8 and 20 weeks after grafting the normal nerve, animals were anesthetized and the sciatic nerve and the tibial nerve above the ankle were exposed. A stimulating electrode (bipolar silver wire) connected to a Grass S88 stimulator (Grass Instruments, Quincy, Massachusetts, U.S.A.) was applied to the sciatic nerve proximally to the graft. The monopolar recording electrode (a glass microelectrode filled with 2 M NaCl and 2% agar) was placed distally on the tibial nerve. The recording electrode was connected to a half-cell (Clark Electromedical Instruments, Reading, U.K.) filled with 2 M NaCl. This was connected to the remote headstage of a Neurolog NL102 DC preamplifier (Digitimer, Welwyn Garden City, U.K.) and then to an AC-DC amplifier (NL106) with the gain set to x 100 and the DC offset always adjusted to 0V. The signal was displayed on a Tektronix 5113 dual-beam storage oscilloscope with a 5A26 differential amplifier (the filters: Hf-3dB set to 1 MHz; Lf-3dB set to DC offset) and a 5B12N time base (Tektronix Inc., Beaverton, Oregon, U.S.A.). A silver-silver chloride reference electrode was placed in the tissue between the two electrodes. The tissues were bathed in phosphate buffered saline and covered with a layer of paraffin oil. The region of the nerve in contact with the stimulating or recording electrodes was gently raised into the paraffin oil layer which acts as an electrical insulator. Monophasic compound action potentials were produced across the graft, using a 0.1-ms pulse and varying the amplitude depending on the threshold of the fibers so that all possible peaks were displayed. The distance between the recording and stimulating electrodes was measured, and the oscilloscope trace photographed to enable the calculation of conduction velocities. The contralateral nerve was used as the control for each animal. Animals were killed by an overdose of Halothane BP (RMB Animal Health Ltd., Dagenham, U.K.), an inhalation anesthetic, after electrophysiology and various tissues were removed.
Muscle weights. The flexor digitorum longus (FDL) muscle which is innervated solely by the tibial nerve was removed from the experimental and contralateral limbs and weighed.
Morphometric analysis. Both the experimental and contralateral tibial nerves were removed in the region of the recording electrode above the ankle. These were cut into 1-mm transverse sections and fixed in 2.5% glutaraldehyde in Sorenson's buffer overnight. After washing in buffer, they were fixed overnight in 1% osmium tetroxide in Sorenson's buffer. The tissue was washed in 70% ethanol, dehydrated through graded ethanol, and embedded in araldite.
Transverse sections (1 µ m) were stained with 1% aqueous paraphenylene diamine (Sigma, Poole, U.K.) for 30 min, rinsed with distilled water, and the background staining was removed by twice immersing in absolute alcohol for 2 min. This stains myelin brown/black and leaves other tissues relatively unstained (6). Morphometric analysis was carried out using a Sight Systems image analysis system (Newbury, Berks, U.K.) connected to a Zeiss Axioskop microscope (Zeis, U.K.) with a x 50 epiplan neofluar objective (for non-coverslip preparations) and an optivar for extra magnification of x 1.6. The analysis was performed on the intensity of gray of a digitized black and white image. The myelin sheaths appeared dark gray while the axons and background appeared lighter. A binary image was formed from this showing only the dark gray areas. The gray level threshold was manually adjusted so that only the myelin was recognized as having sufficient intensity to be measured. The image was manually edited so artifacts were ignored and adjoining myelin sheaths separated. The automated system measured the area of every complete myelin sheath in each field together with the total area enclosed by each myelin sheath. The entire transverse section and, therefore, the entire tibial nerve was analyzed so the total number of myelinated fibers were counted. From the measurements each fiber diameter and myelin sheath thickness was calculated (the fiber was assumed to be circular).
Statistical analysis. Student's t tests were used to compare the conduction velocities of the fastest fiber group of experimental and contralateral nerves and the weights of experimental and contralateral FDL muscles.
RESULTS
Clinical assessment. In the animals injected with M. leprae , the average clinical score at the time of the operation was M2 Sl/2 (weak toe-curling, slow or normal sensory response), indicating that damage to the nerve caused by the intraneural granuloma had occurred. Nerve function in all animals was absent after the operation since the tibial nerve is severed completely in this procedure. The first signs of sensory recovery occurred 4-6 weeks after muscle grafting of the M. /VpraeMnjected nerve, and by 10 weeks some sensory function was present in all animals (Table 2a). Sensory recovery to SI was present at 8 weeks in the 12 recipients of grafts in the normal nerve. One recipient of a graft in the granulomatous nerve was still SI at 20 weeks, and all recipients of grafts in the normal nerve were S2 at 18 weeks.
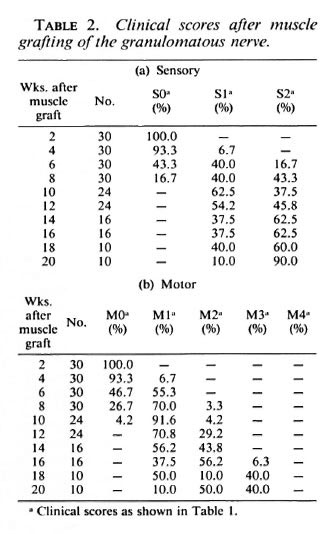
After grafting of the granulomatous nerve, the first signs of motor function return in some animals also occurred at 4-6 weeks (Table 2b), but it was 11 weeks before all animals gave at least an Ml response (resistance to dorsiflexion). By 20 weeks 1 animal was still Ml, 5 were M2 (weak toecurling), and the remaining 4 were M3 (strong toe-curling). In all of the graft recipients in the normal nerve, motor recovery to M1/M2 had occurred by 7 weeks and to M3, by 14 weeks.
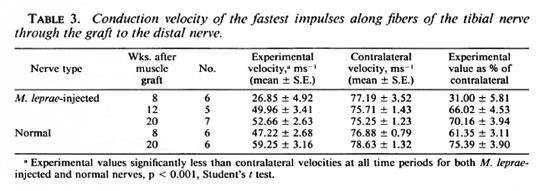
Electrophysiology. In the normal guinea pig tibial nerve, the monophasic recording technique detected at least five peaks within the compound action potential produced. To create an accurate comparison of the functioning of experimental and contralateral nerves, the velocities of the first peak, that is, the fastest conducting fibers in thenerve, were compared (Table 3). The velocities of the fastest fibers of the experimental nerves were significantly (p < 0.001) lower than the contralateral values at all times after grafting of both granulomatous and normal nerves. At 8 weeks after grafting of the granulomatous nerve, the mean conduction velocity of the experimental nerves was 31% of the contralateral value; whereas the mean conduction velocity of the grafted normal nerve was 61% and similar to the mean conduction velocity at 12 weeks after grafting of the granulomatous nerve. In neither group of animals did the conduction velocities of the grafted nerves regain contralateral values by 20 weeks.
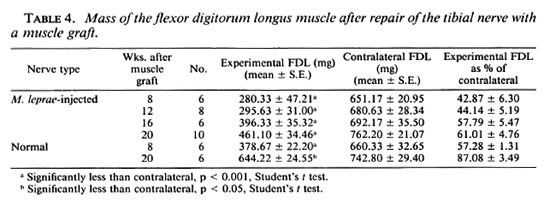
Muscle weights. Although weight loss can be due to lack of use of the limb as well as denervation, the FDL weight gives an indication of functional motor recovery. Increased muscle weights, whether through increased limb movement or reinncrvation, show that regeneration through the graft had occurred. The weights of the FDL innervated by grafted granulomatous nerves showed a gradual increase at each timepoint (Table 4). At 8 weeks the FDL was 43% of the contralateral weight, by 20 weeks it was 61%. The FDL innervated by grafted normal nerves was 57% of the contralateral weight at 8 weeks and 87% at 20 weeks.
Morphometric analysis. The fiber size distribution and number of myelinated axons in the contralateral tibial nerve above the ankle in each group were similar. Fig. 1a shows the data from a representative group of contralateral nerves. The number of myelinated fibers in the tibial nerve was very low at 8 weeks after grafting of an M. leprae nerve (Table 5), with only very small fibers (Fig. lb). The number and size of myelinated fibers increased as more fibers had regenerated to reach this point and those present increased in diameter (Fig. 1, c, d, e and Table 5). At 20 weeks, the myelinated fiber numbers had reached at least a third of the contralateral values (only one nerve with less than 1000 myelinated fibers). Myelinated fiber numbers in the grafted normal nerves at 8 weeks were similar to those in the 12 week grafted granulomatous nerves (Table 5). Their numbers had reached about 60% of contralateral numbers by 20 weeks. The diameters of the majority of the myelinated fibers at 20 weeks in both groups of grafted nerves were less than 10 µ m (Fig. 1, e and g). In contralateral nerves, 55% of the fibers were more than 10 µ m in diameter (Fig. 1, a). In the regenerating nerve fibers the myelin sheath thickness corresponded to the fiber diameter, i.e., thin sheaths around small diameter fibers.

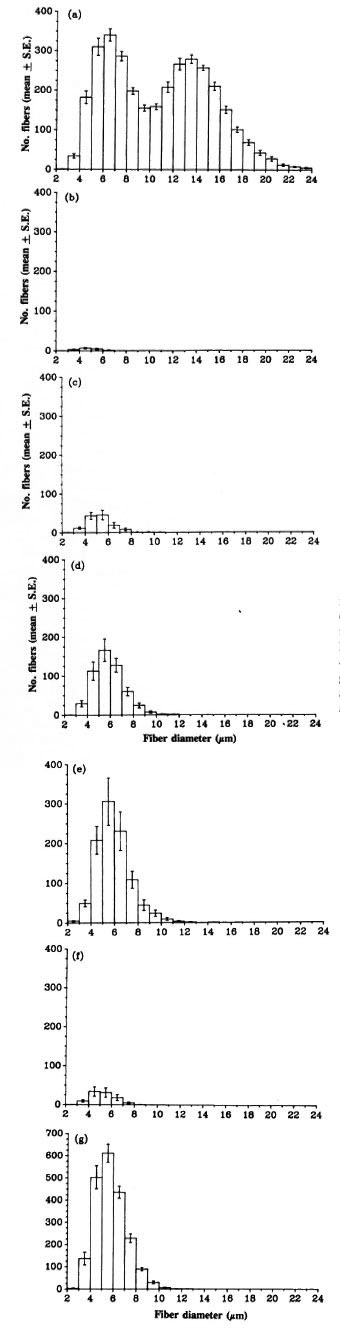
Fig. 1. Fiber size distribution of myelinated axons in the tibial nerve distal to the graft: (a) = contralaterals (N = 11); (b) = 8 weeks after grafting of granulomatousnerve (N = 6); (c) = 12 weeks after grafting of granulomatous nerve (N = 6); (d) = 16 weeks after grafting of granulomatous nerve (N = 6); (e) = 20 weeks after grafting of granulomatous nerve (N = 5); (f) = 8 weeks after grafting of normal nerve (N = 6); (g) = 20 weeks after grafting of normal nerve (N = 6).
DISCUSSION
In this study we have used a guinea pig model to represent some elements of the damage found in human leprosy nerves. It is the most appropriate model available for experimental studies. The guinea pig produces consistent experimental mycobacterial-induced granulomas within 5 weeks of intraneural M. leprae injection. Infiltration of inflammatory cells, edema, perineurial thickening, fibrosis and axonal damage are seen in this model (4). All of these features also occur in leprous neuritis (9). There are some disadvantages to the model. The direct injection of M. leprae organisms breaks the blood-nerve barrier. The M. leprae organisms are not taken into the Schwann cells of the guinea pig. Lastly, the M. leprae is cobalt-irradiated, i.e., it is dead, rather than a metabolically active organism so the damage caused is self-limiting. The data presented in this paper, however, demonstrate that despite the acute nature of the nerve damage, there is sufficient fibrosis in the nerve distal to the granuloma at 5 weeks to slow down regeneration after grafting.
Clinical assessment, which is the least precise measure used, showed earlier recovery of both motor and sensory functions in the recipients of grafts in the normal nerve. This was particularly apparent for the motor function, for which all recipients of the grafts in the normal nerve showed near-normal function by 14 weeks. After repair of the granulomatous nerve, only four animals reached this level of recovery, and that at 20 weeks.
Electrophysiology showed conduction through the graft at increasing velocity as regeneration continued up to and possibly beyond 20 weeks. The conduction velocities at 8 weeks in the grafted normal nerve were similar to those in grafted granulomatous nerves at 12 weeks. In both types of grafted nerve, the conduction velocities at 20 weeks were significantly lower than in the contralateral nerves. This is probably due to the smaller fiber diameter in the regenerated nerves since velocity is related to axon diameter in normal nerves.
The increase in weight of the FDL, which is innervated by no other nerve, suggests that successful rcinnervation of the muscle by the tibial nerve had occurred. The reinnervation of motor end plates correlates with recovery of muscle mass after injury (7). It is important that fibers do not just regenerate through the graft but successfully reinnervate the correct end organs. The fact that both the clinical motor function and the weight of the FDL muscle improved at each timepoint assessed after grafting indicates that reinnervation of this muscle was occurring. Quantitative analysis of motor end plates in the muscle could confirm this and is yet to be carried out. The weight of the FDL innervated by the grafted normal nerves at 8 weeks was similar to the weight of the FDL innervated by grafted M. leprae injected nerves at 16 weeks, again showing earlier regeneration in nongranulomatous nerves.
Morphometry showed that the regeneration of myelinated fibers proceeded more slowly than the functional recovery of the nerve, with few myelinated fibers at the early timepoints. At 20 weeks there were only one third of the fibers of the contralateral in grafted granulomatous nerves and two thirds in the grafted normal nerves. The functioning of the nerves recovered more quickly, as shown by the clinical and electrophysiological results. These results show that there is no need for a complete regeneration of myelinated fibers in order to regain adequate function. It has been reported that up to a third of the fibers in a normal nerve may be lost without noticeable functional loss (l5). A further increase in numbers may be possible over longer time periods.
The advantage of the use of the guinea pig to test this method of nerve repair is that electrophysiological studies on exposed nerves and morphometrical analyses can be carried out which would be impossible to do in humans, where the main assessment after grafting is by clinical means-Single methods of measuring nerve regeneration give limited data as seen if only one of the electrophysiology or morphometry techniques is used to study the 20-week muscle graft in granulomatous nerves (good functional recovery and low numbers of myelinated fibers). By combining the techniques, as in this study, a clearer picture of regeneration is given.
Acknowledgment. This work was supported by the British Leprosy Relief Association (LEPRA). The help of Mr. S.E. Gschmeissner and the technical assistance of Mr. P. Pappasavva and Mr. F. Schindlcr are gratefully acknowledged.
REFERENCES
1. ANTIA, N. H., PANDYA, S. S. and DASTUR, D. K. Nerves in the arm in leprosy. 1. Clinical, electrodiagnostic and operative aspects. Int. J. Lepr. 38(1970)12-29.
2. BOURREL, P. Surgical rehabilitation. (Editorial) Lepr. Rev. 62(1991)241-254.
3. CHAISE, F. and ROGER, B. Neurolysis of the common peroneal nerve in leprosy; a report on 22 patients. J. Bone Joint Surg. [Br.] 67-B(1985)426-429.
4. COWLEY, S. A., BUTTER, C, VERGHESE, S., CURTIS, J. and TURK, J. L. Nerve damage induced by mycobacterial granulomas in guinea pig sciatic nerves. Int. J. Lepr. 56(1988)283-290.
5. DASTUR. D. K., PANDYA, S. S. and ANTIA, N. H. Nerves in the arm in leprosy. 2. Pathology, pathogenesis and clinical correlations. Int. J. Lepr. 38(1970)30-48.
6. ESTABLE-PUIG, J. F., BAUER, W. C. and BLUMBERG, J. M. Technical note; paraphenylene diamine staining of osmium-fixed plastic embedded tissue for light and phase microscopy. J. Neuropath. Exp. Neurol. 24(1965)531-535.
7. FRYKMAN, G. K., MCMILLAN, P. J. and YEGGE, S. A review of experimental methods measuring peripheral nerve regeneration in animals. Orthop. Clin. North Am. 19(1988)209-219.
8. GLASBY, M. A., GSCHMEISSNER, S. G., HITCHCOCK, R. J. 1., HUANG, C. L. H. and DE SOUZA, B. A. A A comparison of nerve regeneration through nerve and muscle grafts in rat sciatic nerve. Neuro-Orthopedics 2(1986)21-28.
9. JOB, C. K. Nerve damage in leprosy. Int. J. Lepr. 57(1989)532-539.
10. NOORDEEN, S. K., LOPEZ-BRAVO, L. and SUNDARE-SAN, T. K. Estimated number of leprosy cases in the world. Bull. WHO 70(1992)7-10.
11. NORRIS, R. W., GLASBY, M. A., GATTUSO, J. M. and BOWDEN, R. E. M. Peripheral nerve repair in humans using muscle autograft; a new technique. J. Bone Joint Surg. [Br.] 70-B(1988)530-533.
12. PEREIRA. J. H., BOWDEN, R. E. M., GATTUSO, J. M. and NORRIS, R. W. Comparison of results of repair of digital nerves by denatured muscle grafts and end-to-end suturing. J. Hand Surg. [Br.] 16B(1991)519-521.
13. PEREIRA, J. H., COWLEY, S. A., GSCHMEISSNER, S. E., BOWDEN, R. E. M. and TURK J. L. Denatured muscle grafts for nerve repair: an experimental model of nerve damage in leprosy. J. Bone Joint Surg. [Br.] 72-B(1990)874-880.
14. PEREIRA. J. H., PALANDE, D. D., SUBRAMANIAN, A., NARAYANAKUMAR, T. S., CURTIS, J. and TURK, J. L. Denatured autologous muscle graft in leprosy. Lancet 338(1991)1239-1240.
15. SHERREN, J. Injuries of Nerves and Their Treatment. New York: Wood, 1907.
16. SUNDERLAND, S. The internal anatomy of nerve trunks in relation to the neural lesions of leprosy; observations on pathology, symptomatology and treatment. Brain 96(1973)865-888.
1. Ph.D.; Departments of Pathology and Anatomy, Royal College of Surgeons of England, London, U.K.
2. Ph.D.; Departments of Pathology and Anatomy, Royal College of Surgeons of England, London, U.K.
3. F.R.C.S.; Departments of Pathology and Anatomy, Royal College of Surgeons of England, London, U.K.
4. M.D., Departments of Pathology and Anatomy, Royal College of Surgeons of England, London, U.K.
Reprint requests to Dr. Jill Curtis, Department of Pathology, Huntcrian Institute, Royal College of Surgeons of England, 35-43 Lincoln's Inn Fields, London WC2A 3PN, U.K.
Received for publication on 24 July 1992.
Accepted for publication in revised form on 7 December 1993.