- Volume 62 , Number 1
- Page: 64–74
Denatured muscle grafts for nerver repair in an experimental model of nerve damage in leprosy. 2. Recovery of peripheral peptide-containing nerves assessed by quantitative immunohistochemical study
ABSTRACT
A marked depiction of neuropeptide-immunoreactive nerves, a consequence of the nerve damage which is commonly found in leprosy, has been reported in peripheral tissues of leprosy patients and of a leprosy animal model. The aim of this study was to investigate peripheral reinnervation following a denatured autologous muscle graft in an animal model of leprosy nerve damage. Possible reinnervation of the foot-pad skin was studied by immunohistochemistry using antisera to the neuronal marker protein gene product 9.5 (PGP), the neuropeptides calcitonin gene-related peptide (CGRP), substance P (SP), vasoactive intestinal peptide (VIP), and the C-flanking peptide of neuropeptide Y (CPON). The extent of the reinnervation process was assessed by image analysis quantification at different time points. At 8 weeks after muscle grafting, there were small numbers of immunoreactive nerves (p < 0.05). At 12, 16, and 20 weeks postoperatively there was a gradual increase in all immunostaining. At 20 weeks, no significant difference was found for PGP-, CGRP-, and SP-immunoreactive nerves in the epidermal and subepidermal layers compared to control (contralateral) tissue. In experimental tissue the recovery of immunoreactive nerves around sweat glands took longer (up to 12 weeks) than in other skin compartments, but after that time the recovery was rapid and at 20 weeks no difference was measured for VlP-immunoreactive nerves in comparison with controls. Around blood vessels, the recovery of CGRP- and CPON-immunoreactive fibers was slow, and at 20 weeks a difference with control samples (p < 0.01) was noted. In the same area, there was no significant difference for PGP immunoreactivity between controls and tissues at 20 weeks. In contrast, the immunoreactive nerve bundles in the dermis showed a faster recovery than nerves in other skin areas, with amounts similar to controls at 20 weeks. The significant recovery of immunorcactive nerves, in particular of those containing sensory neuropeptide, is consistent with the described functional recovery.RÉSUMÉ
Une perte marquée de nerfs immunoréactifs aux neuropeptides, une conséquence des lésions nerveuses fréquemment retrouvée dans la lèpre, a été rapportée dans les tissus périphériques de patients lépreux et d'un modèle animal de la lèpre. Le but de cette étude était d'étudier la réinnervation périphérique après une greffe dénaturée d'un muscle autologue sur un modèle animal ayant une lésion nerveuse de lèpre. La réinnervation du coussinet plantaire a été étudiée par immunochimie en utilisant des antisera vis-à-vis du produit génique protéique 9.5 du marqueur neuronal (PGP), du peptide génique de la calcitonine des neuropeptides (CGRP), de la substance P (SP), du peptide intestinal vasoactif (VIP), et du peptide C du neuropeptide Y (CPON). L'étendue du processus de réinnervation a été évaluée par quantification analytique d'images à différents moments. Huit semaines après la greffe de muscle, il y avait un petit nombre de nerfs immunoréactifs (p < 0.05). Aux douzième, seizième et vingtième semaine après l'opération, il y avait une augmentation progressive de toutes les réactions immunologiques. Après 20 semaines, aucune différence significative n'a été observée pour les nerfs réagissant au PGP, CGRP et SP dans les couches épidermiques et sous-épidermiques par rapport au tissu de controle (contralateral). Dans le tissu de l'expérimentation, la récupération des nerfs immunoréactifs prit plus longtemps autour des glandes sudoríparos (jusqu'à 12 semaines) que dans les autres régions de la peau, mais après ce moment, la récupération a été rapide, et après 20 semaines, on n'a mesuré aucune différence entre les nerfs immunoréactifs au VIP et les controles. Autour des vaisseaux sanguins, la récupération des fibres immunoréactives au CGRP et CPON était lente, et après 20 semaines, on a noté une différence avec les échantillons de controle (p < 0.01 ). Dans la môme zone, il n'y avait pas de différence significative après 20 semaines pour l'immunorcactivité au PGP entre les controles et les tissus. Par contraste, les paquets de nerfs immunoréactifs dans le derme montraient une récupération plus rapide que les nerfs dans les autres régions de la peau, avec une intensité similaire aux controles après 20 semaines. La récupération significative des nerfs immunoréactifs, en particulier ceux contenant du neuropeptide sensoriel, est cohérente avec la récupération fonctionnelle décrite.RESUMEN
Se ha reportado una marcada depleción de nervios inmunorcactivos a neuropeptidos (una consecuencia del daño nervioso comunmente encontrado en la lepra) tanto en los tejidos periféricos de los pacientes con lepra como en un modelo animal de la enfermedad. El objetivo de este estudio fue el investigar la reinervación periférica subsecuente a un injerto autólogo de músculo desnaturalizado en un modelo animal en el que la lepra indujo un daño nervioso. La reinervación de la piel de la almohadilla plantar se estudió por inmunohistoquímica usando antisucros contra las siguientes substancias: el marcador neuronal PGP 9.5 (PGP), los neuropépetidos relacionados con la calcitonina (CGRP), la substancia P (SP), el péptido vasoactivo intestinal (VIP), y el péptido C que flanquea al neuropéptido Y (CPON). El grado del proceso de reinervación se estableció "cuantificando" las imágenes a diferentes intervalos de tiempo. Ocho semanas después del injerto hubieron números muy pequeños de nervios inmunoreactivos (p < 0.05). A las 12, 16, y 20 semanas se notó el incremento gruadual en todas las inmunotinciones. A las 20 semanas no se encontraron diferencias significativas con el tejido control contralateral en cuanto a la reactividad para PGP, CGRP, y SP de los nervios de las capas epidérmica y subepidérmica. En el tejido experimental, la recuperación de los nervios inmunorcactivos alrededor de las glándulas sudoríparas tomó más tiempo (hasta 12 semanas) que en los otros compartimentos de la piel pero después de esc tiempo la recuperación fue rápida y a las 20 semanas no se encontraron diferencias con los controles en cuanto a los nervios VIP-inmunoreactivos. Alrededor de los vasos sanguíneos, la recuperación de las fibras CGRPy CPON-inmunoreactivas fue lenta, y a las 20 semanas se notó una diferencia con las muestras control (p < 0.01). En la misma área, a las 20 semanas no hubo una diferencia significativa de la inmunoreactividad a PGP entre los controles y los tejidos experimentales. En contraste, los haces de nervios inmunoreactivos en la dermis mostraron una recuperación más rápida que los nervios en otras áreas de la piel con números similares a los de los controles a las 20 semanas. La significante recuperación de los nervios inmunoreactivos, en particular de aquellos que contienen neuropeptidos sensoriales, es consistente con la recuperación funcional descrita previamente.Several studies have indicated the presence of peptide-containing nerves in different structures of the human skin (1, 4, 11, 14, 19, 25, 30). The most abundant cutaneous neuropeptides include substance P (SP), calcitonin gene-related peptide (CGRP), vasoactive intestinal polypeptide (VIP), neuropeptide tyrosine (NPY) and its C-flanking peptide (CPON). Nerves immunoreactivc for these neuropeptides appear to be involved in a variety of cutaneous diseases where they show characteristic and quantifiable changes (18, 26, 28).
Nerve damage is a regular feature of the pathology and clinical symptomatology of leprosy (2, 22, 23) . Previous studies have shown marked depletion of neuropeptide-containing nerves in skin biopsies of leprosy patients (15), which reflects the cutaneous sensory and autonomic dysfunctions observed in this disease. Similar neuropeptide changes were also observed in an animal model of leprosy (13).
Recently, a guinea pig model of leprosy neuropathy was established (3). Further studies have shown that the nerve damage could be repaired using muscle autografts, as assessed by the recovery of sensory and motor functions and by histology of the nerve distal to the graft (20). In view of the morphometric and functional results found after muscle grafting for nerve repair (6), it was of interest to determine whether these findings might be paralleled by neuronal changes in the skin, particularly in relation to the repair of sensory and autonomic fibers.
Since there are no immunohistochemical studies of the alterations of neuropeptide-containing nerves in this experimental model, the aim of this investigation was to assess by image analysis quantification the recovery of neuropeptide-containing nerves in the guinea pig foot-pad skin at different times after granuloma excision and grafting with denatured autologous muscle. Antibodies for the general neuronal marker protein gene product 9.5 (PGP) (5, 9) were used to define the distribution of all cutaneous innervation. Specific subpopulations of nerve fibers were identified by their immunoreactivity to the sensory neuropeptides CGRP and SP, and to the autonomic neuropeptides VIP and CPON.
MATERIALS AND METHODS
Animal model
Cobalt-irradiated Mycobacterium leprae organisms (108) suspended in 0.01 ml saline were injected into the tibial fascicle of the right sciatic nerve of 27 guinea pigs (250-300 g) as described previously (6). Autologous muscle grafting was carried out 5 weeks after granuloma induction, as previously described. Groups of animals (at least six per group) were killed at 8, 12, 16 and 20 weeks after muscle grafting.
Immunohistochemistry
Skin from the area of the foot pad innervated only by the tibial nerve was collected from the grafted limb (experimental specimens) and from the contralateral control side. The tissues were fixed by immersion in Zamboni's fluid for 18 hr at 4ºC, washed in phosphate buffered saline (PBS; pH 7.2) containing 15% sucrose and 10 µ g/ml sodium azide, and stored at 4ºC prior to the preparation of cryostat blocks.
Frozen sections (10 µ m thick) were collected onto poly-L-lysine-coated slides and allowed to dry for 1 hr at room temperature. Skin sections were immunostained by an indirect immunofluorescence method (15) using a variety of antibodies (The Table). Serial sections were cut and collected sequentially onto seven slides such that every seventh section was stained for the same antigen. At least four sections were stained with each antiserum.
Quantitative evaluation of immunoreactive fibers
Quantification was carried out on the control and experimental tissue of animals killed at 8 and 20 weeks after grafting, as these were the first point of electrophysiology and morphometrical analysis, and the longest time point, respectively (6). In all animals, samples from both experimental and contralateral (control) sides were evaluated.
Immunoreactive nerves were quantified in the different skin areas according to their distribution and functional significance. The selected skin compartments were as follows:
Epidermis and papillary dermis. CGRP and SP immunoreactivities were evaluated in these areas since these peptides are known to subserve sensory functions at the dermoepidermal junction and control the regulation of papillary blood vessel tone.
Sweat glands. Imunoreactivities for CGRP and VIP were quantified in the nerve plexus around the sweat glands.
Hypodermal blood vessels. Nerves immunoreactive for CGRP and CPON, which are known to modulate vasomotor tone, were evaluated in the adventitial plexus.
Hypodermal nerve bundles. To evaluate the growth of regenerating nerve fibers CGRP, SP, CPON, and VIP immunoreactivities were measured in nerve bundles.
General topography of nerves. To evaluate the general topography of the nerves, PGP antibodies were used and the staining quantified in all areas of the skin.
Quantification was carried out using a method previously described (26). In brief, a low-light video camera (Panasonic MV1900) was mounted on a Vanox fluorescence microscope (Olympus, U.K.) and linked to a VIDAS analyzer (Kontron, U.K.). A long pass filter around 560 nm was used to reduce autofluorescence. The field image was defined by use of interactively defined frames in order to establish precisely the area to be measured while deleting any unwanted part of the image present on the screen. The image of immunostained structure was enhanced to increase the signal/ noise contrast. A background substraction procedure was carried out by taking the maximum grey value of background as the threshold at which to segregate the image. The binary image obtained in this way was then measured according to the selected parameters.
The chosen parameters of measurement were total field fluorescence area ( µ m2), intercept counts, and field counts of the immunoreactive nerves. For each immunostaining in every specimen, two random sections were analyzed and in each section three fields (x 20 objective magnification) were measured for every area as defined above. The data are presented as the mean values of the total counts for each skin compartment. The statistical analysis of the results was carried out using analysis of variance with logged data to ensure that the normality assumption of the ANOVA was valid. Multiple t tests were used to compare the values at different time points for each nerve population. The p values quoted have been adjusted using the Bonferroni method.
RESULTS
Immunohistochemistry
The quantitative results of immunofluorescence area, intercept counts and field counts gave consistent results when control and experimental tissues were compared. Hence, a single description of the quantitative results which refers to all parameters is given. A full statistical analysis was carried out between the mean values of PGP immunoreactivity of the controls, 8-week and 20-week timepoints, and the analysis of variance showed significant heterogeneity (p < 0.001). For the neuropeptide-immunoreactive nerves, a statistical comparison could be carried out only between the controls and experimental sides at 20 weeks. In experimental tissue at 8 weeks, only a few immunoreactive fibers were observed in some sections, while most of the measured fields gave a negative result.
Epidermis/papillary dermis
Numerous PGP-immunoreactive fibers were seen in the epidermis and papillary dermis of control samples (Fig. 1 a) while a moderate amount of CGRP- and SP-immunoreactivc nerve were observed, particularly around the capillaries of the papillary plexus (Fig. 1 b).
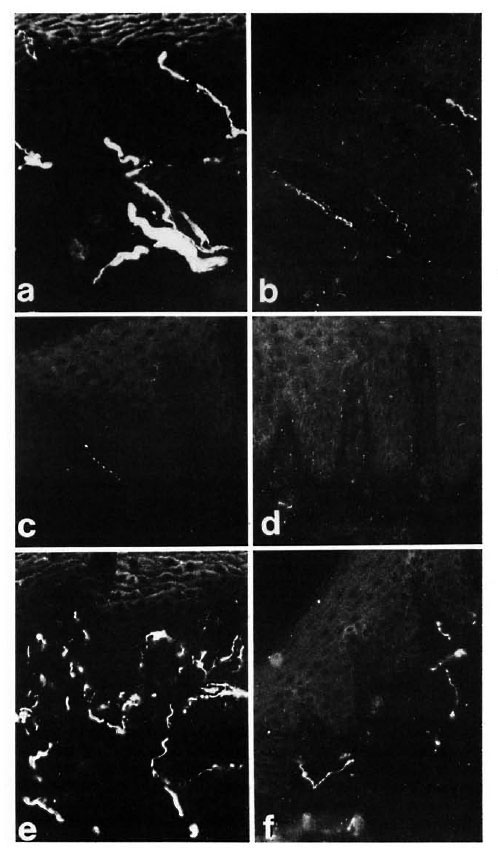
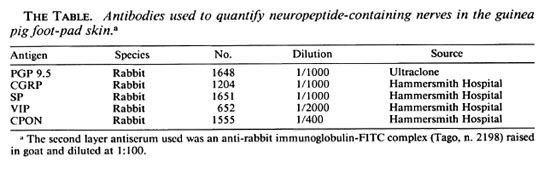
Fig. 1. Dermo-epidermal nerve plexus from foot-pad skin of a control specimen showing (a) abundant PGP immunoreactive fibers and (b) fewer CGRP-immunoreactive nerves. The same skin area at 8 weeks after muscle graft: (c) few POP immunoreactive fibers are seen; (d) the CGRP-immunoreactive fibers have disappeared completely. At 20 weeks after grafting, a significant recovery was found for (e) PGP-immunoreactive and (f) CGRP-immunoreactive nerves. Indirect immunofluorescence method (all x 320).
At 8 weeks after grafting, few PGP-immunoreactive nerves were present in the epidermis and papillary dermis (Fig. 1 c), and quantitative assessment showed a significant decrease of PGP-immunoreactive nerves (p < 0.01) (Fig. 2a). CGRP- and SP-immunoreactive fibers were sharply decreased in the epidermis and around the microvcssels in the papillary dermis (Fig. 1 d).
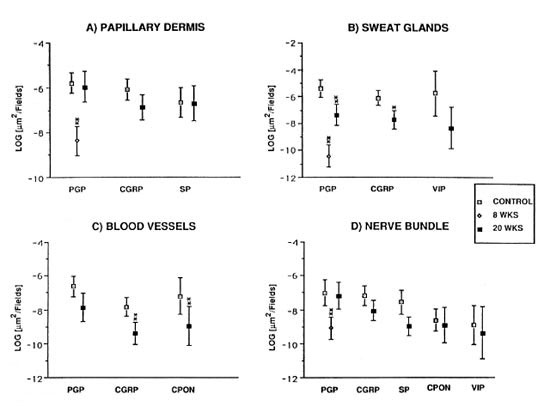
Fig. 2. Diagrams showing the variations of PGP, CGRP, SP, CPON and VIP immunoreactive area ( µ m2) per field in different skin compartments. All data are expressed as mean values with the S.E.M. represented by error bars. (A) Epidermis and papillary dermis: no measurement could be made for CGRP and SP at 8 weeks after grafting since these types of nerves were almost completely absent (**p < 0.01). (B) Sweat glands: nomeasurement could be made for CGRP and VIP at 8 weeks after grafting (*p < 0.05; **p < 0.01). (C) Dermal blood vessels: no measurement could be made for any of the immunoreactivities at 8 weeks (**p < 0.01). (D) Dermal nerve bundles: no peptide immunoreactivity could be measured at 8 weeks after grafting (**p < 0.01).
At 12 weeks postoperatively, some degree of recovery was seen for all immunoreactivities when compared to samples at 8 weeks, indicating that at this point reinnervation of the skin can be detected by immunohistochemistry. The number of immunoreactive nerves increased progressively with time, and at 20 weeks all immunoreactive nerves appeared widely distributed to both epidermis and papillary dermis (Fig. 1, e and f). Quantitative results showed no significant difference for PGP, CGRP and SP immunoreactivities between the controls and the 20-week samples (Fig. 2a).
Sweat glands
In control samples, numerous nerves immunoreactive for PGP, CGRP and VIP were found around the sweat glands (Fig. 3a). Following muscle graft, CGRP- and VIP-immunoreactive nerves were absent at 8 weeks postoperatively (Fig. 3b). The situation remained unchanged up to 12 weeks, followed by a rapid increase, particularly for VIP-immunoreactive nerves, to nearly normal levels by 20 weeks (Fig. 3c).
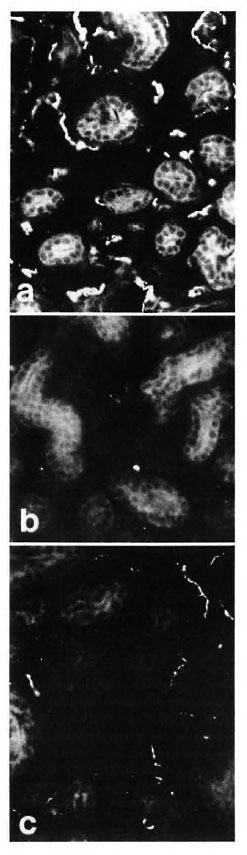
Fig. 3. (a) = Abundant VIP immunoreactive fibers are observed around the secretory portions of sweat glands in control specimens. (b) = At 8 weeks after muscle graft the VIP immunoreactive fibers are totally depleted. (c) = A significant recovery of VIP immunoreactive fibers around sweat glands was observed 20weeks after muscle grafting. Indirect immunofluorescence method (all x 310).
Quantification showed a difference for PGP when comparing controls versus 8 weeks and controls versus 20 weeks (p < 0.01) (Fig. 2b), and for CGRP between the controls and 20-week groups (p < 0.05). No statistically significant difference was found for VIP-immunoreactive nerves between the controls and the 20-week groups (Fig. 2b).
Blood vessels
In control samples, moderate amounts of PGP-, CGRP- and CPON-immunoreactive nerves were observed around dermal and hypodermal blood vessels (Fig. 4 a). At 8 weeks postoperatively no immunoreactivity for CGRP or CPON was observed (Fig. 4 b). A certain amount of recovery was seen at 20 weeks (Fig. 4 c), but the immunoreactive fibers were not as numerous as in the control samples. These results were consistent with those of quantification, which showed a difference between the controls and 20-week muscle grafts for CGRP (p < 0.01) and CPON (p = 0.003), but no significant difference between the two groups for PGP (Fig. 2 c).
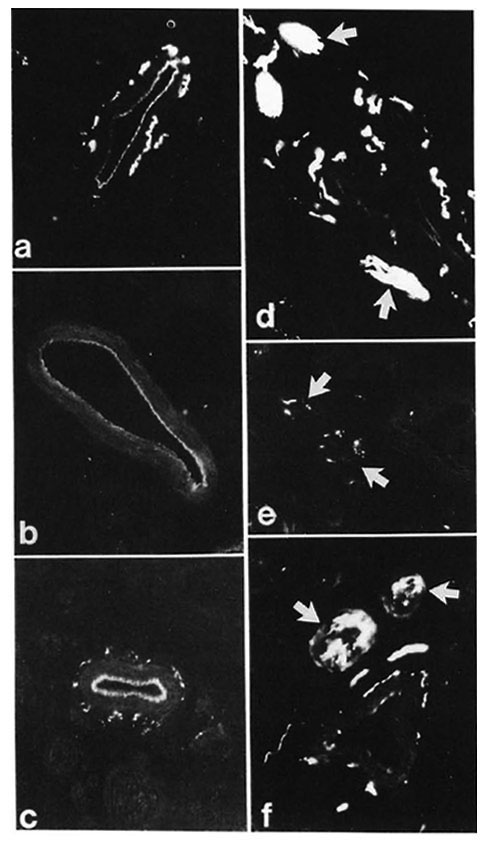
Fig. 4. (a) = Control specimen: CPON immunoreactive fibers in the adventitial plexus from a hypodermal blood vessel. (b) = Complete disappearance of adventitial CPON immunoreactive nerves is found at 8 weeks after muscle grafting. (c) = At 20 weeks after graft, the CPON immunoreactive fibers innervating a hypodermal blood vessel show a significant recovery. (d) = Hypodermal nerve bundles in the dermis with intense PGP immunoreactivity (arrows) are seen in a control specimen. (e) = Marked decrease (arrows) can be seen in the number of PGP immunoreactive fibers in hypodermal nerve bundles at 8 weeks after muscle grafting. (f) = A significant recovery of PGP immunoreactive fibers from hypodermal nerve bundles (arrows) is found at 20 weeks after graft. Indirect immunofluorescence method (a, b, c x 380; d, e, f x 300).
Hypodermal nerve bundles
These structures showed a strong immunoreactivity for PGP (Fig. 4 d) in the control samples. A moderate number of all types of immunoreactive fibers was observed within nerve bundles in the dermis. Following muscle grafting, the immunoreactivities for the markers and the neuropeptides showed a similar pattern of low levels at 8 weeks (Fig. 4 e) followed by an increase. However, their recovery was faster than those observed in other skin areas, being already evident at 16 weeks. At 20 weeks after muscle graft the immunoreactive nerves showed no major difference to those in the control tissues (Fig. 4 f). This pattern of change was reflected in the quantification results for all immunoreactivities which showed a difference for PGP between the controls and 8-week groups (p < 0.01) but no significant difference between the controls and the 20-week groups for all immunoreactivities (Fig. 2d).
DISCUSSION
This study shows low numbers of PGPand neuropeptide-immunoreactive fibers in guinea pig foot-pad skin at the first timepoint following muscle grafting, which is indicative of an incomplete reinnervation process in the peripheral tissues. Neuropeptide changes were particularly evident for CGRP- and SP-immunoreactive nerves in the papillary dermis, for CGRP- and VIPimmunoreactive fibers around sweat glands, and for CGRP- and CPON-immunoreactive fibers innervating blood vessels. At later timcpoints, there was a gradual reinnervation by all nerve types in most skin areas, consistent with the results of morphometrical analysis, electrophysiology and func-tional recovery (6).
In this study, quantitative immunohistochemistry was of value since it allowed an objective determination of the neural changes at the different timepoints. This was particularly important when assessing the extent of immunoreactive nerve recovery in the different skin compartments since it can be difficult to assess, by subjective microscopical examination, possible differences between samples with abundant numbers of nerves, such as are observed in control and experimental tissues at 20 weeks after grafting.
Interestingly, the distribution pattern of neuronal terminals after reinnervation is similar to that of the control tissue. At present it is not entirely clear what mechanism is regulating this phenomenon, but it has been suggested that the reinnervation process in denervated tissue might follow the existing pathway of degenerated nerves (8). At 8 weeks after grafting, some recovery of immunoreactivity was evident for PGP, while only a few neuropeptide-immunoreactive nerves were observed. This is probably due to the fact that a recovery of the nerve terminal structure, as indicated by PGP immunostaining, might have started at this time, although the transport of neuropeptides from the neuronal cell body to the terminal was still affected. Hence, it is likely that nerve function in relation to the studied neuropeptides may still be impaired. However, it cannot be excluded that other neuropeptides/neurotransmitters, which were not identified in this study, may be present in the nerve terminals and might be responsible for some functional response as observed in some animals at 8 weeks (6).
The recovery of immunoreactivities for neuronal markers and neuropeptides did not occur within the same timeframe in all skin compartments. At 20 weeks after muscle grafting, quantification of perivascular PGPimmunoreactivc fibers showed considerable recovery with all measured parameters, and no statistical difference was seen between control and experimental sides. However, the recovery of CGRP- and CPON-immunoreactive nerves was not complete at 20 weeks, as demonstrated by quantification. This would indicate complete structural recovery, but a slower recovery related to neuropeptide functions. By contrast, nerve bundles in the dermis and nerve fibers in the cpidermal/subepidermal layer showed a similar degree of recovery for all immunoreactivities. The recovery of nerve bundles appeared at an earlier time than for nerves in other areas, since the bundles represent the initial pathway of nerve regeneration before the fibers branch out into single terminal fibers.
It is interesting to note that 20 weeks after grafting a more complete nerve recovery was found for sensory nerves at the dermo-epidermal junction. This might be related to the possible action of neurotrophic factors secreted by the keratinocytes (10, 24, 29, 31) since these substances may improve the nerve fiber growth in this area. All of the guinea pigs harvested at 20 weeks in this experiment showed a normal response to the crude sensory test used (6). Autonomic immunoreactive nerves related to sweat glands and blood vessels showed a slower recovery, which is parallel to the slow recovery of motor nerve functional tests after muscle grafting (6, 20). It also has been noted in other reinnervation experiments that sensory CGRP-immunoreactive nerves are the first to appear (12, l7). Similarly, in fetal skin CGRP-immunoreactive nerves are the first neuropeptide-containing fibers to be detected, soon after the appearance of PGP-immunoreactive nerve (27). It is thought that CGRP may have a trophic role (7) and contribute to the healing and repair processes (16), hence actively contributing to the reinnervation process.
A recent pilot study has shown that the muscle-grafting technique applied to leprosy patients produces a considerable improvement of sensory perception (21). These results in man are consistent with the experimental evidence in the animal model showing a return to normal amounts of sensory neuropeptide-containing nerves in dermo-epidermal junction after long-term muscle grafts. The partial recovery of CGRP, CPON and VIP immunoreactive fibers, innervating blood vessels and sweat glands, indicates that the vasomotor and secretory functions also can be improved by muscle grafting, although a longer time than for sensory nerves might be needed for full recovery.
Acknowledgment. This work was supported by LEPRA, The Colt Foundation, and The Grand Charity of Freemasons. LS was supported by Grant BE90-062 from the Ministerio de Educación y Ciencia, Spain. The authors arc grateful to Deborah Price, Medical Physics Department, Hammersmith Hospital, for statistical analysis of the data.
REFERENCES
1. BJORKLUND, H., DALSGAARD, C. J., JONSSON, C. E. and HEMRANSSON, A. Sensory and autonomic innervation of non-hairy and hairy human skin; an immunohistochemical study. Cell Tissue Res. 243(1986)51-57.
2. CHAROSKY, C. B., GATTI, J. C. and CARDAMA, J. E. Neuropathies in Hansen's disease. Int. J. Lepr. 51(1983)576-586.
3. COWLEY, S. A., BUTTER, C, VERGHESE, S., CURTIS, J. and TURK, J. L. Nerve damage induced by mycobacterial granulomas in guinea pig sciatic nerves. Int. J. Lepr. 56(1988)283-290.
4. DALSGAARD, C. J., JONSSON, C. E., HOKFELT, T. and CUELLO, A. C. Localization of substance P immunoreactivc nerve fibres in human digital skin. Experientia 39(1983)1018-1020.
5. DALSGAARD, C. J., RYDH, M. and HAEGERSTRAND, A. Cutaneous innervation in man visualized with protein gene product 9.5 (PGP 9.5) antibodies. Histochemistry 92(1989)385-389.
6. DE BLAQUIERE, G. E., CURTIS, J., PEREIRA, J. H. and TURK, J. L. Denatured muscle grafts for nerve repair in an experimental model of nerve damage in leprosy. 1. An electrophysiological and morphometric study. Int. J. Lepr. 62(1994)55-63.
7. DENIS-DONINI, S. Expression of dopaminergic phenotypes in the mouse olfactory bulb induced by the calcitonin genc-rclatcd peptide. Nature 339(1989)701-703.
8. DIAMOND, J. and JACKSON, P. C. Regeneration and collateral sprouting of peripheral nerves. In: Nerve Repair and Regeneration, Its Clinical and Experimental Basis. St. Louis: C. V. Mosby, 1980.
9. GULBENKIAN, S., WHARTON, J. and POLAK, J. M. The visualization of cardiovascular innervation in the guinea pig using an antiserum to protein gene product 9.5 (PGP 9.5). J. Auton. Nerv. Syst. 18(1987)235-247.
10. HARPER, S. and DAVIES, A. M. NGF mRNA expression in developing cutaneous epithelium related to innervation density. Development 110(1990)515-519.
11. JOHANSSON, O. A detailed account of NPY-immunoreactive nerves and cells of human skin; comparison with VIP-Substance P and PHI containing structures. Acta Physiol. Scand. 128(1986)147-153.
12. KARANTH, S. S., DHITAL, S., SPRINGALL, D. R. and POLAK, J. M. Reinnervation and neuropeptides in mouse skin flaps. J. Auton. Nerv. Syst. 31(1990)127-134.
13. KARANTH, S. S., SPRINGALL, D. R., KAR, S., GIBSON, S. J., ROYSTON, J. P., BANNERJEE, D. K. and POLAK, J. M. Time related decrease of substance P and CGRPin central and peripheral projections of sensory neurons in Mycobacterium leprae infected nude mice: a model for lepromatous leprosy in man. J. Pathol. 161(1990)335-345.
14. KARANTH, S. S., SPRINGALL, D. R., KUHN, D. M., LEVENE, M. M. and POLAK, J. M. An immunocytochemical study of cutaneous innervation and the distribution of neuropeptides in man and commonly employed laboratory animals. Am. J. Anat. 191(1991)369-383.
15. KARANTH, S. S., SPRINGALL, D. R., LUCAS, S., LEVY, D., ASHBY, P., LEVENE, M. M. and POLAK, J. M. Changes in nerves and neuropeptides in skin from 100 leprosy patients investigated by immunocytochemistry. J. Pathol. 157(1989)15-26.
16. KIARTANSSON, J. and DALSGAARD, C. J. Calcitonin gene-rclated peptide increases survival of musculocutaneous llap in the rat. Eur. J. Pharmacol. 142(1987)355-358.
17. MANEK, S., TERENGHI, G., SHUREY, C, NISHIKA-WA, H., GREEN, C. J. and POLAK, J. M. Neovascularization precedes neural changes in the rat groin skin flap following denervation: an immunohistochemical study. Br. J. Plastic Surg. (1992) in press.
18. NAUKHARINEN, A., NICKOLOFF, B. J. and FARBER, E. M. Quantification of cutaneous sensory nerves and their substance P content in psoriasis. J. Invest. Dermatol. 92(1989)126-129.
19. O'SHAUGHNESSY, D. J., MCGREGOR, G. P., GHA-TEI, M. A., BLANK, M. A., SPRINGALL, D. R., GU, J., POLAK, J. M . and BLOOM, S. R Distribution of bombesin, somatostatin, substance-P and vasoactive intestinal polypeptide in feline and porcine skin. Life Sci. 32(1983)2827-2836.
20. PEREIRA, J. H., COWLEY, S. A., GSCHEMEISSNER, S. E., BOWDEN, R. E. M. and TURK, J. L. Denatured muscle grafts for nerve repair; an experimental model of nerve damage in leprosy. J. Bone Joint Surg. [Br.] 72B(1990)874-880.
21. PEREIRA, J. H., PALANDE, D. D., SUBRAMANIAN, A., NARAYANAKUMAR, T. S., CURTIS, J. and TURK, J. L. Denatured autologous muscle graft in leprosy. Lancet 338(1991)1239-1240.
22. SHETTY, V. P. Nerve damage in leprosy. Int. J. Lepr. 56(1988)619-621.
23. SHETTY, V. P., ANTIA, N. H. and JACOBS, J. M. The pathology of early leprous neuropathy. J. Neurol. Sci. 88(1988)115-131.
24. SOBUE, G., YASUDA, T., MITSUMA, T., ROSS, A. H. and PLEASURE, D. Expression of nerve growth factor receptor in human peripheral neuropathies. Ann. Neurol. 24(1988)64-72.
25. TAINIO, H., VAALASTI, A. and RECHARDT, R. The distribution of sympathetic, adrenergic, tyrosine hydroxylase and neuropeptide Y-immunoreactive nerves in human axillary sweat glands. Histochemistry 85(1986)117-120.
26. TERENGHI, G., BUNKER, C. B., LIU, Y-F., SPRING-ALL, D. R., COWEN, T., DOWD, P. M. and POLAK, J. M. Image analysis quantification of peptideimmunoreactivc nerves in skin of patients with Raynaud's phenomenon and systemic sclerosis. J. Pathol. 164(1991)245-252.
27. TERENGHI, G., SUNDARESAN, M., MOSCOSO, G. and POLAK, J. M. Neuropeptides and a neuronal marker in cutaneous innervation during human foetal development. J. Comp. Neurol. (1992) in press.
28. VAALASTI, A., SUOMALAINEN, H. and RECHARDT, L. Calcitonin gene-related peptide immunoreactivity in prurigo nodularis: a comparative study with neurodermatitis circumscripta. B. J. Dermatol. 120(1989)619-623.
29. WALICKE, P. A. Novel neurotrophic factors, receptors and oncogenes. Ann. Rev. Neurosci. 12(1989)103-126.
30. WALLENGREEN, J., EKMAN, R. and SUNDLER, F. Occurrence and distribution of neuropeptides in the human skin. Acta Dermatol. Venereol. 67(1987)185-192.
31. YAAR, M., GROSSMAN, K., ELLER, M. and GIL-CHREST, B. A. Evidence for nerve growth factormediated paracrine effects in human epidermis. J. Cell. Biol. 115(1991)821-828.
1. M.D.; Department of Histochemistry, Royal Postgraduate Medical School, Hammersmith Hospital, London, U.K.
2. Ph.D.; Department of Histochemistry, Royal Postgraduate Medical School, Hammersmith Hospital, London, U.K.
3. D.Sc, Department of Histochemistry, Royal Postgraduate Medical School, Hammersmith Hospital, London, U.K.
4. Ph.D.; Department of Pathology and Anatomy, Royal College of Surgeons of England, London, U.K.
5. B.Sc; Department of Pathology and Anatomy, Royal College of Surgeons of England, London, U.K.
6. F.R.C.S.; Department of Pathology and Anatomy, Royal College of Surgeons of England, London, U.K.
7. M.D., Department of Pathology and Anatomy, Royal College of Surgeons of England, London, U.K.
Reprint requests to Dr. G. Terenghi at his present address: Blond Mclndoe Centre, Queen Victoria Hospital, East Grinstead, Sussex RH 19 3DZ, U.K.
Received for publication on 24 July 1992.
Accepted for publication in revised form on 7 December 1993.