- Volume 62 , Number 1
- Page: 75–88
Water soluble complexes of C14 and C16 fatty acids and alcohols in media for cultivation of leprosy-derived psychrophilic mycobacteria
ABSTRACT
Host-grown Mycobacterium leprae cell suspensions oxidized water-soluble complexes of palmitic acid, myristic acid, cetyl alcohol, and myristyl alcohol prepared with randomly methylated-β-cyclodextrin as host molecules. Gas chromatography analysis showed that the water-soluble complexes retained their chemical structure following sterilization in the autoclave. Bioavailability of the two long-chain fatty acids and the corresponding long-chain alcohols was confirmed by Warburg manometric techniques with host-grown M. leprae cell suspensions. Inoculated with host-grown M. leprae cells in chemically well-defined, simple liquid and agar media, acid-fast bacilli were cultivable in primary cultures and subcultures at 10ºC with (NH4)2S04 as the N source and water-soluble palmitic acid, myristic acid, cetyl alcohol or myristyl alcohol as the C and potent energy sources.M. phlei oxidized the complexed palmitic acid and myristic acid but not cetyl alcohol or myristyl alcohol. On agar media with any of these four carbon sources and (NH4)2S04 but not ammonium thioglycolate as the N source, M. phlei grew abundantly at 36ºC. In liquid media only myristyl alcohol supported growth of M. phlei without any growth with palmitic acid, cetyl alcohol or myristic acid.
The leprosy-derived, cold-loving cultures ("M. psychrophilum") were not fully tested for classification and identification. The cells are strongly acid-fast facultative psychrophiles, adapted in subcultures to mesophilic growth. They grow in chemically well-defined media with 14 and 16 C long-chain fatty acids or alcohols as the C and energy sources. None of the cultures grow on Lowenstein or 7H9 media. Heat-killed suspensions of the 4th and 6th subcultures provoke Mitsuda-type late skin reactions in tuberculoid, borderline and borderline-tuberculoid but not in lepromatous leprosy volunteers. When grown with (NH4)2S04 as the N source (but not with the reducing agent ammonium thioglycolate) the subcultures multiplied abundantly in the foot pads of mice.
It became evident that leprosy-derived, facultative psychrophilic mycobacteria really exist. Mycobacteria of this cluster do not distinguish between 14 or 16 C long chains with COOH or CH2OH as terminal bindings. Cells are quite aerophilic and grow preferentially on agar slant surfaces. This is probably due to the abundant 02 requirement of each palmitic-acid molecule to produce 129 ATP molecules. The reducing agent, ammonium thioglycolate, was toxic in culture media for M. phlei. "M. psychrophiluin" lost infectivity with ammonium thioglycolatc as the N source in the media.
RÉSUMÉ
Des suspensions cellulaires de Mycobacterium leprae mises en croissance chez un hôte ont oxydé des complexes solubles dans Peau d'acide palmitique, d'acide myristique, d'alcool cétylique et d'alcool myristylique préparé avec comme molécule-hôte du β-cyclodextrin méthylé aléatoirement. Une analyse par chromatographic gazeuse a montré que les complexes hydrosolubles conservaient leur structure chimique après stérilisation dans l'auloclave. La biodisponibilité des deux acides gras à longue chaîne et des alcools à longue chaîne correspondants a été confirmée par des techniques manométriques de Warburg avec des suspensions cellulaires cellulaires de M. leprae mises en croissance chez un hôte. Inoculés avec des cellules de M. leprae mises en croissance chez un hôte dans des milieux chimiquement bien définis de liquide simple et d'agar, des bacilles acido-résistants étaient cultivables dans des cultures primaires et des sous-cultures à 10ºC avec du (NH4)2S04 comme source d'azote et de l'acide palmitique, de l'acide myristique, de l'alcool cétylique ou myristylique hydrosolubles comme source de carbone et d'énergie.M. phlei oxyda les complexes d'acide palmitique et d'acide myristique, mais non l'alcool cétylique ni l'alcool myristylique. Sur milieu d'agar, avec une quelconque de ces quatre sources de carbone et du (NH4)2S04 mais pas du thioglycolate d'ammonium comme sourec d'azote, M. phlei s'est multiplié abondamment à 36ºC. En mileu liquide, seul l'alcool myristylique stimulait la croissance de M. phlei, alors qu'il n'y avit aucune croissance avec l'acide palmitique, l'alcool cétylique ou l'acide myristique. Les cultures dérivées de la lèpre et aimant le froid ("M. psychophrylum") n'ont pas été entièrement analysées pour la classification et l'identification. Les cellules sont fortement acido-résistantes et psychrophilcs de manière facultative, adaptées à la croissance mésophile dans des sous-cultures. Elle croissent dans des milciux chimiquement bien définis avec des acides gras ou des alcools à longue chaîne de 14 ou 16 atomes de carbone comme sources de carbone et d'énergie. Aucune de ces cultures ne poussait sur milieu de Lowenstein ou 7H9. Des suspensions tuées par la chaleur des quatrièmes et sixièmes sous-culturcs provoquent des réactions cutanées tardives de type Mitsuda chez des patients volontaires présentant une lèpre tuberculoide, borderline et borderline tuberculoide, mais non chez ceux présentant une lèpre lépromatcuse. Quand elles étaient mises en culture avec du (NH4)2S04 comme source d'azote (mais pas avec l'agent réducteur thioglycolate d'ammonium), les sous-cultures se multipliaient abondamment dans les coussinets plantaires des souris.
II est devenu evident que des myobactéries dérivées de la lèpre, psychrophilcs facultatives, existent réellement. Les mycobactérics de ce type ne font pas la distinction entre des longues chaînes à 14 ou 16 atomes de carbone terminées par un complexe COOH ou CH2OH. Les cellules sont relativement aérobiques et se multiplient préférentiellement sur des surfaces d'agar inclinées. Ceci est probablement dû au besoin abondant d'oxygène de chaque molécule d'acide palmitique pour produire des molécules d'ATP. L'agent réducteur, le thioglycolate d'ammonium, était toxique pour M. phlei dans le milieu de culture. "M. psychrophilum''' perdait son infectivité avec du thioglycolate d'ammonium comme source d'azote dans le milieu de culture.
RESUMEN
Las suspensiones del Mycobacterium leprae cultivado in vivo oxidaron los complejos hidrosolubles del ácido palmítico, del ácido mirislico, del alcohol cetilico, y del alcohol mirislico preparados con beta-ciclodextrinas mediadas al azar como moléculas acarreadoras. Los análisis por cromatografía de gases mostraron que los complejos hidrosolubles retuvieron su estructura química después de su esterilización en el autoclave. La bioacecsibilidad de los 2 ácidos grasos de cadena larga y sus correspondientes alcoholes fue confirmada por la técnica manométríca de Warburg utilizando suspensiones de M. leprae cultivado in vivo. Se peparó un medio químicamente definido (liquidoo con agar) el cual contenía sulfato de amonio como fuente de nitrógeno y los derivados hidrosolubles del ácido palmítico o del alcohol mirístico como fuente de carbono y de energía. Estos medios se inocularon con M. leprae propagado in vivo y se incubaron a 10ºC. M. phlei oxidó los complejos de ácido palmítico y de ácido mirístico pero no los complejos de alcohol cetílico o mirístico. Además de que creció abundantemente a 37ºC en los medios con agar con cualquiera de las 4 fuentes de carbono y sulfato de amonio, pero no cuando se ultilizó tioglicolato de amonio como fuente de nitrógeno. En medio líquido, sólo el alcohol mirístico permitió el crecimiento del M. phlei y no hubo ningún crecimiento con ácido palmítico, con alcohol cetílicoo con ácido mirístico.Los cultivos psicrofílicos de "M. psychroplulum" aislados de casos de lepra no fueron estudiados de manera suficiente como para establecer su clasificación c identificación inequívocas. Aunque estas bacterias ácido resistentes son psicrófilos facultativos, son adaptables por subcultivo a su crecimiento mesofílico. Crecen en medios químicamente definidos con ácidos grasos o alcoholes de 14 y 16 carbonos como fuente de carbono y energía. Ninguno de los cultivos crece en medio de Lowcnstcin o 7H9. Las suspensiones inacti vadas por calor del 4º y 6º subculti vos provocan reacciones tardías de tipo Mitsuda en pacientes con lepra tubereuloide y tuberculoide subpolar pero no en pacientes con lepra lepromatosa. Cuando se cultivan con sulfato de amonio como fuente de nitrógeno (pero no con tioglicolato de amonio) los subcultivos recuperados se multiplican abundantemente en las almohadillas plantares del ratón. Se demostró la existencia real de las micobacterias psicrofílicas facultativas derivadas de la lepra. Las micobacterias de este grupo no distinguen entre las cadenas largas de 14 ó 16 carbones con extremos COOH o CH2OH terminales. Las células son muy acrofilicas y crecen preferentemente sobre superficies inclinadas de agar. Esto se debe probablemente al abundante requerimiento de oxígeno de cada molécula de ácido palmítico para producir 129 moléculas de ATP. El agente reductor tioglicolato de amonio fue tóxico en los medios de cultivo para cl M. phlei. "M. psychrophilum"pendió su infectividad cuando se usó tioglicolato de amonio como fuente de nitrógeno en el medio.
Franzblau (8) reported oxidation of palmitic acid with increased synthesis of energy-rich phosphate (ATP) and of phenolic glycolipid-I (PGL-I) by Mycobacterium leprae . This result opened a new avenue to the in vitro cultivation of M. leprae by identifying a fatty acid as a carbon and energy source for M. leprae . As such, this fatty acid is an excellent candidate to be incorporated into culture media to grow the hitherto noncultivable causative agent of leprosy. Ishaque (11) confirmed the findings of Franzblau and provided evidence that the oxidation of palmitic acid occurs via β -oxidation. As shown by Wheeler, et al. (27), all of the enzymes for β -oxidation are present in M. leprae .This mechanism is responsible for potent energy generation. Palmitic acid is one of the richest sources of energy, since one molecule, when fully oxidized through several metabolic pathways in the mitochondria, yields 129 molecules of ATP (18).
Once the practical importance of the above results was recognized cultivation trials were initiated with palmitic acid in prospective culture media. Only crude homogenization was obtained with β -cyclodextrin added to the aqueous system, but the fatty acid precipitated into a solid state following sterilization in the autoclave. The turbid media were inoculated with host-grown M. leprae cells, and the cultivation of psychrophilic mycobacteria was reported (l4-17).
This observation indicated that palmitic acid was biologically available from the solid state. Indeed it is known that cells are able to scavenge solid substrates from the environment. It was, however, imperative to develop crystal clear liquid or semisolid media containing palmitic acid in order to achieve maximal bioavailability of the hydrophobic fatty acid. Preparation of transparent media was also necessary in order to visualize the multiplication of cells with the naked eye or photometrically.
Taking advantage of the rapid development in the application of substituted cyclodextrins (CD) in biotechnology (l6, 17, 21, 24), we are now able to report the preparation of culture media with water-soluble complexes of not only palmitic acid but also the corresponding, structurally related, longchain alcohols.
With these techniques at hand we investigated the growth-promoting effect of substrates other than but structurally closely related to palmitic acid. Here we report that the C14 fatty acid (myristic acid) and the corresponding alcohols C16 (cetyl alcohol) and C14 (myristyl alcohol) complexes have a growth-promoting activity higher than palmitic acid on leprosy-derived psychrophilic mycobacteria.
The facultative psychrophilic-mesophilic growth of a leprosy-derived cluster of mycobacteria is a most unusual observation. The detailed and convincing data of Binford (5) and Brand (6) concerning "the association between damage from leprosy and temperature" were also considered in our further cultivation trials. Binford and Brand clearly showed that the morbid anatomy of leprosy occurs exclusively in cooler parts of the body. The question thus arose, how cool is cool enough for M. leprae to grow axenically?
The purpose of the present investigations was to provide further data in our efforts toward in vitro cultivation of M. leprae , as well as to satisfy the authors' curiosity as to whether or not a hitherto unknown psychrophilic cluster of mycobacteria really existed.
MATERIALS AND METHODS
M. leprae . Host-grown cells were obtained from the foot pads of athymic nude (Nu) mice. These were supplied by Dr. M. Ishaque (Institut A. Frappier, Université du Québec, Quebec, Canada). Semipurifted suspensions were prepared as previously described (13). M. leprae -infected armadillo lepromas, livers and spleens were obtained from Dr. E. Storrs (Medical Research Institute, Melbourne, Florida, U.S.A.). The M. leprae cells were separated from pooled, heavily infected tissues (liver, spleen and subcutaneous lepromas) of an M. leprae -infected armadillo. Semipurified suspensions were prepared (13). Cell suspensions contained 24 mg wet weight/ml, calculated by weighing the heavily packed sediment of semipurified suspensions centrifuged at 12,000 x g for 15 min at 4ºC.
Armadillo- and Nu-mice-derived cell suspensions were inoculated into 7H9 and Lowenstein media and incubated for 60 days at 36ºC in order to detect the presence of leprosy-derived cultivable mycobacteria.
"Mycobacterium psychrophilum ."Cultures and subcultures were isolated from M. leprae -infected foot pads of Nu mice over the past 6 years. Cells were grown and maintained on palmitic acid semisolid agar medium at 10ºC (14-17).
M. phlei . Cultures were grown in Sauton liquid medium for 10 days at 37ºC. Cells were collected and separated by centrifugation. The sediment was washed twice with excess of M/15 phosphate buffer (pH 6.5) and homogenized with a Potter-Elwehyem hand grinder. Washed again with phosphate buffer, the suspension was centrifuged and the sediment weighed. Cells were resuspended in the buffer so as to obtain 40 mg wet weight of cells per ml.
Chemicals. Sterile solutions of water-soluble complexes of palmitic acid, myristic acid, cetyl alcohol, and myristyl alcohol contained 10 mg/ml of the acids or alcohols, respectively, in 40% randomly methylated β -cyclodextrin (RAMEB-CD; Cyclolab R. D. Laboratories, Budapest, Hungary) in distilled water.
Middlebrook ADC enrichment solution (BBL1 1887-ADC) was purchased from Becton Dickinson and Co., Cockeyville, Maryland U.S.A.
Substrate oxidation. Substrate oxidation was measured in the Warburg apparatus under air at 32ºC in the usual manner (19). There was added to each Warburg vessel a bacterial suspension of 24 mg wet weight of M. leprae or 40 mg wet weight of M. phlei , respectively, per 1.0 ml of M/15 phosphate buffer (pH 6.4) in the main compartment and 1.0 ml of M/15 phosphate buffer (pH 6.4) containing different amounts of the tested substrates in the side arm. To the center wall insert 0.2 ml of 10% KOH was added with folded analytical filter paper. The equilibrium period was 15 to 20 min at which time the content of the side arm was tipped into the main compartment. Oxygen uptake was measured manometrically at the time intervals shown in Tables 1 and 2. Control vessels contained buffer without the substrates in the side arm, or buffer without the cells in the main compartment.
Basal solution. In 500 ml distilled water were dissolved KH2P04 , 4.5 g; Na citrate, 1.0 g; MgS04, 0.05 g; ferric ammonium citrate, 0.05 g; and (NH4)2S04, 2.5 g.
Liquid culture media. Water-soluble palmitic acid solution (6 ml) was pipetted into a beaker. This solution contained 10 mg/ml palmitic acid. With constant magnetic stirring 94 ml 10% w/v RAMEB-CD solution in distilled water was added slowly. The clear solution was thus made to a final volume of 100-ml basal solution. This procedure was necessary to avoid replacing the hydrophobic substrates with H2O molecules in the enclosure complexes.
Ten ml of the solution was distributed into each of 50-ml screw-cap tubes and autoclaved for 25 min at 110ºC at 15 lbs pressure.
Liquid culture media were also prepared by replacing palmitic acid with cctyl alcohol, myristic acid, or myristyl alcohol water-soluble complexes.
When cooled to room temperature, 0.5 ml BBL11886-ADOC (containing serum albumin fraction V-dextrose, oleic acid, and catalase) was pipetted aseptically into each tube of liquid media.
Semisolid agar media. To 500 ml of basal solution, 500 ml of distilled water and 30 g of granulated agar was added and dissolved at 80º-90ºC with occasional stirring. The clear agar solution was distributed while hot into 50-ml screw-cap tubes, sterilized in the autoclave for 25 min, and cooled to room temperature in an inclined position to make agar slants.
One ml of the previously prepared, sterile, complete liquid medium was pipetted aseptically into each of the agar slants. The agar slants with the added palmitic acid, cetyl alcohol, myristic acid, or myristyl alcohol liquid media respectively were placed (for 24 hr) at room temperature in an inclined position in order to impregnate the agar surface with the ingredients of the liquid media.
Inoculation of media, incubation and estimation of growth. All cultivation trials were done with M. leprae suspensions originating from foot pads of mice. Liquid media were inoculated from standardized suspensions in primary cultures and in subcultures so as to have a cell count as close as possible to 3.5-4.0 x 105 acid-fast bacilli (AFB) per ml. Solid media were inoculated with 0.1 ml of a heavy suspension, counting close to 107 cells per 0.1 ml. This bacterial suspension was placed carefully on the surface of the agar slant. The inoculum was gently homogenized on the agar surface with a 5-mm diameter loop dipped into the liquid medium previously pipetted into the agar medium. This manipulation ensured homogenization of the cells with the nutrient liquid on the agar surface. Once a week during incubation each agar slant was gently tilted once, permitting the liquid to flow over the inoculated slants without washing off the inoculum. Subcultures were obtained from small, well-developed colonies or from the difTuse surface growth by transferring one full 3-mm diameter loopful of cells to a virgin agar slant and homogenizing them on the agar surface as for the primary culture. Transfer from subculture to subculture was made when a well-developed colony or diffuse surface growth was visible with the naked eye, usually at 50-60-day intervals.
In the liquid media the number of AFB/ml of the culture was counted according to Hanks, et al. (10). The samples were withdrawn from the liquid cultures following a 10-sec vigorous shaking on a Vortex Jr. homogenizer.
Growth on the semisolid agar media was easily detected by the increased bacterial mass and colony formation on the surface, as compared to the control tubes inoculated with heat-killed M. leprae cells.
For estimating growth on the agar slants, each primary culture or subculture with each of the tested substrates was inoculated on 4 to 5 agar slants. At 0, 30 and 60 days following inoculation the total number of cells were counted in one of the randomly selected cultures as shown in Table 3. Cells were carefully washed off with 5 ml of 0.9% sterile saline solution from each of the agar slants, using an 18-gauge, 12-cm-long needle attached to a 10-ml syringe. The suspension was then homogenized, declumped, and diluted with saline as required for counting (10).
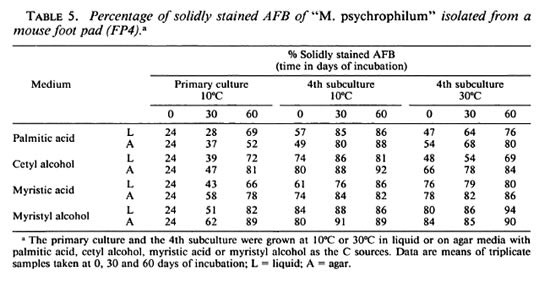
Percentage of solidly stained AFB was counted microscopically on siliconized slides following Ziehl-Neelsen staining of thin smears of the cell suspensions after declumping with chloroform (10) from liquid and from semisolid cultures and subcultures at incubation time intervals as shown in Table 5.
Examination for acid fastness. The cultures in liquid media were inspected regularly for changes of turbidity. Since an increase in turbidity indicated growth, smears were prepared and stained for acid fastness. It was necessary to use siliconized slides to avoid washing ofT the cells due to the detergent effect of the liquid media. The CDs and long-chain alcohols have marked surface-tension-reducing capacity since they are mild detergents (22, 23, 25). Using a 5-mm diameter loop a drop of well-shaken cultures was placed on a siliconized slide and covered with a pctri dish to prevent evaporation. Twenty-five min later the liquid phase of the specimen was siphoned off with a syringe and a 26-gauge needle. Cells at this time were sedimented and electrostatically attached to the siliconized surface of the slide. Fixation of the dried specimen was achieved by placing the air-dried slide on the cover of a boiling water bath for 1 min to avoid caramelization of CD and heat precipitation of the components of the specimen. If this procedure was not observed, staining resulted in the washing off of cells and confusing black spots microscopically. It also was imperative to perform coldstaining of the specimen for 25 min with carbol fuchsin Ziehl-Neelsen stain covering the slides.
Smears from the cultures on semisolid agar were made on regular glass slides following the procedures as for the liquid media cultures.
Mitsuda-type skin reactions. From the 4th and 6th subcultures on palmitic-acid agar media of a culture grown at 10ºC, the cells were washed off and separated by centrifugation and washed three times with excess of 0.15 M phosphate buffer (pH 6.5). The packed sediment was resuspended in 0.9% physiological saline solution containing 0.25% phenol. The suspension was then standardized by adjusting cell counts (10) to obtain 107/0.1 ml AFB. The suspensions were distributed and sealed into vials and autoclaved at 115ºC at 15 lbs of pressure for 40 min. The antigens so prepared were sent to Dr. Enzo Melchior, Jr., University of São Paulo, Department of Dermatology, Ribeirão Preto, Brazil. The antigens were tested on lepromatous, borderline and tuberculoid leprosy patients who volunteered for intradermal, late, Mitsuda-type skin reaction tests. These results are shown in Table 6.
Animal inoculation. Swiss albino mice were divided into groups of six and inoculated into both hindfoot pads with a tuberculin syringe and 36-gauge needle. Each culture was injected into a group of six mice. Into each hindfoot pad 5 x 103 AFB were injected in a 0.05 ml volume. Cells collected from the 4th subculture of the surface of agar slants were washed once with excess of M/15 phosphate buffer (pH 6.5) and resuspended in the buffer following counting (10). Animals were killed 14 months after inoculation. At this time the number of AFB were counted in the scmipurified suspensions of the homogenized, pooled, foot-pad connective tissues. Cultures used for testing infectivity in the mouse foot pads are shown in Table 7.
RESULTS
Oxidation of substrates by M. leprae. Table 1 shows the effect of water-soluble palmitic acid, cetyl alcohol, myristic acid, and myristyl alcohol, respectively, on the respiration of armadillo-derived M. leprae cells. With both the C16 long-chain palmitic acid and its corresponding C16 long-chain cetyl alcohol and the C14 long-chain myristic acid and its corresponding C14 long-chain myristyl alcohol, there was a markedly stimulated oxygen uptake by the host-grown M. leprae cells. Increase in oxygen consumption was considerably higher with the C14 fatty acid and C14 alcohol as compared to the C16 longchain acid and alcohol. The host substance (RAMEB) of the substrate complexes did not have any effect on the endogenous respiration of M. leprae cells. Each of the substrates was tested at 24-hr intervals using the same cell suspension. The obtained values of 02 uptake are, therefore, not absolute but sufficiently accurate when comparing average substrate per endogenous 02 uptake expressed in µ l/4-hr respiration.
Oxidation of substrates by M.phlei. Water-soluble palmitic acid and myristic-acid complexes considerably stimulated 02 uptake by the M.phlei suspension. The corresponding long-chain alcohols, cetyl alcohol and myristyl alcohol, respectively, had an inhibitory effect on oxygen uptake as expressed by an arbitrary indexation of substrate/control affected 02 uptake. The experiment was performed simultaneously with the four test substances and the RAMEB cyclodcxtrin, and the results are shown in Table 2. When the experiment was repeated several times with different batches of the water-soluble complexes, comparable results were obtained; stimulation with the long-chain fatty acids and lower than endogenous respiration with the long-chain alcohols.
Leprosy-derived cultivable mycobacteria. Two colonies of AFB developed on one of the three Lowenstein media inoculated with a loop full of armadillo-derived, heavy M. leprae suspensions after 4 weeks of incubation at 37ºC. Cultures were identified as M. avium-intracellulare . Media inoculated with cell suspensions from the foot pads of mice and from the Warburg vessels remained sterile for 60 days.
Primary cultures in liquid media inoculated with host-grown M. leprae . At 10ºC incubation, AFB multiplied in the presence of both fatty acids and both of the corresponding long-chain alcohols. The exact quantitative estimation of growth was not feasible because of technical difficulties. It became evident that even from the surface of siliconized slides many, if not most, of the bacilli were washed off during staining. Furthermore, the carbol fuchsin stain formed a disturbing dark precipitate with the complexed substrates, thus making accurate counting impossible. Contrast phase microscopical techniques left no doubt of multiplication with all of the four tested substrates. During more than 30 days of incubation at 10ºC a coprecipitation of bacilli with substrates occurred. This further disturbed estimation of growth. Consequently, further cultivation trials were made on semisolid agar media rather than in the liquid media.
Primary culture and subculture on agar slants inoculated with host-grown M. leprae cells. The inoculum from M. leprae suspensions isolated from Nu mice grew slowly but abundantly at 10ºC on the semisolid agar slants impregnated with water-soluble complexes of palmitic acid, cetyl alcohol, myristic acid, or myristyl alcohol. Growth became visible with the naked eye, using a x 6 magnifier at ± 15 days' incubation, as a diffuse, colorless, thin layer and as smooth colorless-to-pale-pinkish colonies with the aging of the cultures. The bacterial mass slowly increased in size during 40 to 60 days of incubation. With all four substrates, the bacilli were strongly acid fast and arranged in small-to-large clumps. Because of the uneven amount of the heavy inoculum, it was difficult to estimate visually any quantitative differences in growth between cultures with the four substrates. However, for the examiner's trained eye there was no doubt that the earliest diffuse growth and colony formation occurred in the presence of myristyl alcohol.
None of the cultures grew on the above agar media at 32ºC or in 7H9, Lowenstein or Dubos media at 36ºC.
Table 3 presents the multiplication of the AFB as counted in suspensions washed off from the surface of agar slants at 0, 30 and 60 days' incubation at 10ºC in the primary cultures. Data are not absolute numbers of bacteria on the slants because of incomplete removal of growth from the agar surface and also because dcclumping of growth with chloroform is not a perfect procedure. The data shown are the means of triplicate samples from serial dilutions and are comparable since the samples were always taken in the same manner. The results show considerable multiplication of the inoculum.
Counting was not performed on subcultures because growth on the agar slants was so obvious to the trained eye that time-consuming counting seemed to be unnecessary.
Growth of M. phlei in liquid and/or agar media. Ten-day-old M. phlei cultures collected from Sauton liquid media grown at 36ºC were homogenized, washed twice with excess M/15 phosphate buffer (pH 6.5), and transferred to liquid and agar media containing the two fatty acids or the two longchain alcohols. A series of media contained (NH4)2S04 or ammonium thioglycolate as the nitrogen source, respectively. In the liquid media the growth was visibly heavier in the presence of myristyl alcohol compared to growth with cetyl alcohol as the carbon source. M. phlei did not grow in liquid media containing palmitic acid or cetyl alcohol with either of the two N sources. On the surface of the agar slants there was a heavy growth with (NH4)2S04 as the nitrogen source with all four substrates as the C source. If ammonium thioglycolate was used as the N source, growth of M. phlei was negligible with any of the four C sources. Whenever growth was registered, the colonies on the agar surface or floating on the surface of liquid media were colorless but became increasingly yellow pigmented with growth, just as on the Sauton media. These results are summarized in Table 4.
Solidly stained bacilli in primary cultures and subcultures of the leprosy-derived " M. psychrophilum . The percentage of solidly stained bacilli in the primary culture and the 4th subculture in liquid and solid media incubated at 10ºC, as well as the 4th subculture at 32ºC, was counted at 0, 30, and 60 days of the incubation period. The percentage of solidly stained AFB was as low as 24% in suspensions of leprosy bacilli freshly isolated from foot pads of mice. As the cells multiplied in the liquid and agar primary cultures, the percentage of solidly stained bacilli increased progressively with time and reached close to 90% in 30 to 60 days. As cultures became adapted to mcsophilic growth at 30ºC the percentage of solidly stained bacilli increased with time. The staining properties of the AFB (as presented in Table 5) were registered in liquid and solid media with (NH4)2S04 as the N source and with any of the four substrates as the C sources. Data in Table 5 are means of triplicate samples.
Mitsuda-type late skin reactions. The standardized suspensions of " M. psychrophilum "antigens as described above were autoclavcd for 40 min at 120ºC at 15 lbs of pressure. Dr. Enzo Melchior, Jr. and Prof. L. M. Bechelli (Department of Dermatology, University of São Paulo at Ribeirão Preto) tested the antigens on the skin of human volunteers for late Mitsuda-type skin reactions. As summarized in Table 6, the heat-killed suspensions gave negative late skin reactions in all of the 16 LL cases similar to that obtained by authentic human lepromin. In 19 lepromatous, borderline, borderline tuberculoid and tuberculoid cases the late skin reactions were all similar to those obtained by authentic human lepromin.
Multiplication of AFB in foot pads of mice. Table 7 shows that there was no multiplication of acid fast cells in the foot pads inoculated with the 4th subculture of leprosy-derived " M. psychrophilum " grown at 10ºC with ammonium thioglycolate as the N source and the 14 and 16 carbon fatty acids or alcohols as the C sources. Fourteen months following inoculation of the foot pads with close to ± 5 x 103 cells grown under the same conditions, with the same C sources, but with (NH4)2S04, as the sole source of N in the media, the number of AFB in the foot pads increased from ± 5x 103 to 2.3 x 105 to 2.4 x 106, as countedin the homogenized soft tissues at the siteof inoculations.
DISCUSSION
The majority of biochemical processes involve an enzyme-catalyzed transformation of a substrate in aqueous medium. The main difficulties which used to arise are: a) substrates such as fatty acids and long-chain alcohols were hydrophobic, sparingly or scarcely soluble in water; b) enzyme or the enzyme-producing microbial cells were sensitive to the toxic effects of the substrate or to inhibitors which could be the product of the transformation; and c) the substrate or the product was unstable under the conditions of the enzymic transformation.
Cyclodextrins form complexes with fatty acids effectively solubilizing the acids in aqueous media. By forming these complexes, cylcodextrins also protect microbial cells from the possible toxic effects of free fatty acids.
Significance of water-soluble lipid complexes. A high proportion of the dry weight of mycobacteria, M. leprae in particular, is lipidic material. Wheeler and coworkers (26, 28 ) investigated and reported lipid metabolism and fatty-acid biosynthesis in hostgrown M. leprae .It became evident that investigations into the cultivation of M. leprae should be focused on developing media which could promote the vital lipid biosynthetic processes necessary for growth and multiplication in axenic conditions. The effect of long-chain fatty acids on the growth and metabolism of mycobacteria has been the subject of various investigations, as already reviewed by Saz (20) in 1949. However, only recently a long-chain fatty acid, palmitic acid, was identified by Franzblau and coworkers (8, 9) as the carbon and energy source in vital processes of M. leprae .These authors also provided important data concerning biophysical optima for the metabolism of M. leprae . These results must be considered in developing prospective culture media to grow the elusive M. leprae , rather than working with empirically designed media as has been done for over a century.
The C14 and C16 fatty acids as well as the corresponding alcohols, myristyl alcohol and cetyl alcohol, can be complexed as guest molecules with hydrophilic host molecules and transformed into water-soluble molecules. The Figure shows this physical encapsulation of palmitic acid as a guest molecule into three DIMEB-CD host molecules. The so-complexed fatty acid is then water soluble. According to its shape, size and molecular configuration, three molecules of the host molecule can encapsulate one molecule of the guest molecule, palmitic acid. Since the preparation of the water-soluble inclusion complexes of lipophiles is a rather simple technology and the methylated CDs do not possess microbiological effects (3), the present solubilization technique seems to be of practical significance. Gas chromatography of the complexed fatty acids and longchain alcohols shows that these substrates retain their chemical structure after sterilization in the autoclave-a further advantage for the preparation of sterile culture media.
The figure. Schematic of the water-soluble complex formation of palmitic acid. A chemically modified dimethyl- β -cyclodextrin (DIMEB) forms a complex with a long-chain hydrophobic fatty acid. The encapsulated hydrophobe guest molecule becomes soluble in water.
The question thus arises as to whether or not the complexed soluble palmitic acid is biologically as active as the insoluble compound. It is known that in the presence of excess water or of a third component the dissociation of the complex takes place and the continuous release of the molecularly dispersed guest substance is ensured. This unique method of release of an active substance results in an improved bioavailability (21, 22). Recently, Ishaque (12) reported that the water-soluble complexes of palmitic-acid salts are oxidized by M. leprae at the same rate as the palmitates insoluble in water. The latency period of oxidation was considerably shorter with the soluble complexes compared to the palmitates insoluble in water. Ishaque also described many advantages of the soluble palmitates versus the hydrophobic forms when proposed for cultivation trials or metabolic studies.
In 1949 Saz (20) already had found that several strains of mycobacteria oxidized long-chain alcohols. He found that the O2 uptake by the tested strains in the Warburg apparatus was tripled with added cetyl alcohol compared to endogenous respiration. This C16 alcohol-structurally closest to palmitic acid-had the maximal substrate oxidation compared to other long-chain alcohols. Indeed, as shown in Table 1, hostgrown M. leprae cells not only oxidized both palmitic acid and myristic acid but also the structurally related alcohols, cetyl and myristyl alcohol. The highest level of O2 uptake was registered with myristyl alcohol as the substrate. These results show that M. leprae does not distinguish between the 16 and 14 carbon fatty acids or the 16 and 14 carbon alcohols as a source of oxidizable substrate. The fact that the four tested substrates as the exclusive sources of carbon promoted axenic growth of the leprosy-derived psychrophilic mycobacteria is strong evidence that the substrates are used as carbon and energy sources as shown in Table 3. The shorter-chain (C14) substrates were better oxidized and promoted better growth than did the C16 substrates, whether acid or alcohol. This may be due to the easier bioavailability or membrane penetration by the somewhat smaller molecule.
An unexplained observation resulted from testing the fast-growing M. phlei for substrate oxidation and in vitro growth with the four substrates. It has been postulated that biological oxidation of a substrate by a microorganism would also promote axenic growth of the same cells by providing carbon and energy sources. The results in Tables 2 and 4 show that such expectations are not necessarily valid, and are not applicable to microorganisms in general. Table 2 shows that respiration of the M. phlei suspension was inhibited quite strongly by cetyl alcohol and myristyl alcohol. Both substrates, incorporated as the sole sources of carbon and energy in a simple, chemically well-defined medium, resulted in heavy growth on the agar slant, and myristyl alcohol promoted growth in the liquid media. These results are experimental evidence that respiration and growth are distinct phenomena, at least for M. phlei , and probably for other microorganisms also. This lesson now learned must be considered when searching for growth-promoting substrates using substrate oxidation techniques.
SH group compound in media. Previously, we reported (13) that host-grown M. leprae and M. lepraemurium oxidized SH group compounds. Since sulfur is required for the formation of CoA thioester and acetyl CoA intermediates in fatty-acid metabolism, we included thioglycolates, e.g., ammonium thioglycolate, as the N and SH sources in our media while attempting to grow M. leprae . Moreover, we thought the reducing effect of the same compound to be useful in lowering 02 tension in the media. This working theory was reconsidered since we learned from Franzblau and Harris (9) that metabolic activity of M. leprae "was negatively affected by strong reducing agents" like thioglycolate. Our findings confirm the results of Franzblau and Harris. As shown in Table 4, thioglycolate completely inhibited the growth of M. phlei in myristyl alcohol liquid media and strongly inhibited its growth on agar slants with several C sources.
Furthermore, Table 7 shows that thioglycolate in the media completely abolished the infectivity of " M. psychrophilum ." It also became evident that S sources other than SH groups can contribute to growth when (NH4)2S04 replaced thioglycolate in the media.
Mitsuda-type skin reactions to heat-killed cultures. With Dr. Enzo Melchior's kind permission, late skin reactions to heat-killed subcultures of leprosy-derived " M. psychrophilum " in 35 human volunteers are presented. The fact that these antigens gave negative late reactions in all LL cases and that these late reactions were similar to those obtained with authentic human lepromin suggests that the antigenic properties of " M. psychrophilum " are close or identical to M. leprae .
Some biological properties of CDs. The CDs were not oxidized by M. leprae and M. phlei , and did not promote growth of these mycobacteria. CDs stimulated the growth of slow-growing Bordetella pertussis by replacing blood in media. ( B. pertussis is very susceptible to inhibitors, e.g., palmitic acid.) When 0.5 µ l/ml of DIMEB was added to the system, increased cell growth was observed (2). Bar and Roken (3) found stimulation of fermentation by CDs, but not as a carbon source. RAMEB-CD is quite resistant not only to enzymatic but to alkaline and acidic degradation, and is also resistant to elevated temperature up to carmelization (23, 24). CDs, mainly the methylated CDs (DIMEB, RAMEB), stimulate fatty-acid synthesis by several mycobacteria (4, 18). Chemical hydrolysis of fats, on the other hand, is enhanced by methylated CDs (7). While small amounts of fatty acids promote in vitro growth of mycobacteria, bovine albumin, serum or usually bovine scrum albumin fraction V is added to the culture media to neutralize toxicity of fatty acids. CDs substitute serum albumin in media and inhibit toxicity of fatty acids and accumulated toxic substances and inhibitors in several cultures (1, 23, 24). In mammalian cell cultures and human interferon production, fatty acid-CD complexes completely substitute for the bovine serum albumin (1).
These properties of CDs must be considered in cultivation trials of hard-to-grow mycobacteria in general and M. leprae in particular.
Bacteriology of the cultivated organism. The results presented here indicate that the cultivated organisms are probably closer to what we call M. leprae than any of the previously described leprosy-derived mycobacteria (LDM). If these cultures are but one of a new cluster of LDM, they merit special attention. So far none of the known LDM except " M. psychrophilum " induces the Mitsuda-type late skin reaction; these cultures differ from LDM in pathogenicity and physical requirements for growth at optimal low growth temperature. In psychrophilic conditions, the cultivated organisms not only multiplied abundantly, but the morphological quality of the acid fast cell population improved considerably during growth.
Solidly stained cells possess a physiological viability and replication potential. Percentage of solidly stained bacilli increased with time in the primary cultures and subcultures attaining up to 89%-95% at the end of week 8 of incubation at 10ºC, but they also adapted to mesophilic growth at 30ºC. The highest percentage of solid staining was counted with myristyl alcohol as the C and energy source in the chemically defined media. These results are strong evidence for multiplication of AFB in the axenic cultures since cell divisions occur only from the solidly stained live bacilli.
The existence of mycobacteria preferring growth temperatures much lower than body temperature became evident once again. The fact that the cultures grow in chemically simple but well-defined media, preferentially on the surface of agar slants with plenty of 02 available for a high energy substrate as the C source at low incubation temperature in primary cultures, adapted in subcultures to mesophilic growth, are characteristics of novelty for students of microbiology.
Acknowledgment. This study was supported by grants from the Cardinal Léger Institute Against Leprosy. The German Leprosy Relief Association generously covered expenses for fine chemicals. Dr. Eleanor Slorrs (Florida Institute of Technology) deserves gratitude for tirelessly supplying M. leprae -infected armadillo tissues. Nu mouse foot pads were donated by Dr. M. Ishaque (Institut A. Frappicr, Université du Québec, Montreal).
We acknowledge with gratitude the expert cooperation of Dr. Enzo Mclchior, Jr. and Prof. L. M. Bechelli in performing and evaluating the late skin reaction to " M. psychrophilum " antigens.
REFERENCES
1. AJINOMOTO Co. Medium for animal tissue culture. Jpn. Kokai 82(194-195).
2. AOYAMA, T., MURASE, Y., IWATA, T, IMAIZUMI, A., SUZUKI, Y. and SATO, Y. Comparison of bloodfree medium (cyclodextrin solid medium) with Bordct-Gangou medium for clinical isolation of Bordetella pertussis .J. Clin. Microbiol. 23(1986)1046-1048.
3. BAR, R. and ROKEM, J. Cyclodextrin-stimulated fermentation of prodigiosin by Serratia macerens . Biotechnol. Lett. 12(1990)447-448.
4. BERGERON, A. R., MACHIDA, Y. and BLOCK, K. Complex formation between mycobacterial polysaccharides or cyclodextrins and palmitoyl coenzyme. J. Biol. Chem. 250(1975)1223-1230.
5. BINFORD, C. H. Comprehensive program for inoculation of human leprosy into laboratory animals. Public Health Rep. 71(1956)955-956.
6. BRAND, P. Association between damage from leprosy and temperature. Int. J. Lepr. 26(1958)423-424.
7. CHEN, J. P. Enhancement of enzymic hydrolysis rate of olive oil in water by dimethyl-cyclodextrin. Biotechnol. Lett. 11(1989)633-636.
8. FRANZBLAU, S. G. Oxidation of palmitic acid by Mycobacterium leprae in an axenic medium. J. Clin. Microbiol. 26(1988)18-21.
9. FRANZBLAU, S. G. and HARRIS, E. Biophysical optima for metabolism of M. leprae . J. Clin. Microbiol. 26(1988)1124-1129.
10. HANKS, J. H., CHATTERJEE, B. R. and LECHAT, M. F. A guide to the counting of mycobacteria in clinical and experimental materials. Int. J. Lepr. 32(1964)156-167.
11. ISHAQUE, M. Direct evidence for the oxidation of palmitic acid by host-grown Mycobacterium leprae .Res. Microbiol. 140(1989)83-93.
12. ISHAQUE, M. Water soluble palmitic acid-methylated cyclodextrin complex; a substrate oxidized by Mycobacterium leprae .Int. J. Lepr. 60(1992)279-280.
13. ISHAQUE, M. and KATO, L. Oxidation of substrates by host-grown mycobacteria cultured from human, armadillo and murine lepromas. Int. J. Lepr. 45(1977)120-131.
14. KATO, L. Psychrophilic mycobacteria in M. leprae -infected tissues. Int. J. Lepr. 56(1988)631-632.
15. KATO, L. Adaptation of Mycobacterium psychrophilum (L) to mcsophilic growth on water-soluble palmitic acid complex agar media. Int. J. Lepr. 60(1992)662-663.
16. KATO, L., SZEJTLI, J. and SZENTE, L. Water soluble complexes of palmitic acid and palmitates for metabolic studies and cultivation trials of M. leprae . Int. J. Lepr. 60(1992)105-107.
17. KATO, L., SZENTE, L. and SZEJTLI, J. Water soluble palmitic acid-cyclodextrin complex in media for cultivation of leprosy-derived psychrophilic mycobacteria. International Cyclo-dcxtrin Symposium, 22-25 April 1992, Chicago, U.S.A. Minutes. Hedges, A. R., cd. Paris: Editions de Sante,1992, pp. 373-376.
18. LEHNINGER, A. L. Biochemistry .2nd edn. New York: Worth Publishers Inc., 1975, p. 580.
19. Machida, Y., Bergeron, R., Flick, P. and Bloch, K. J. EfTccts of cyclodextrins on fatty acid synthesis. J. Biol. Chem. 248 (1973) 6246-6247.
20. SAZ, A. K. The effect of long-chain carbon compounds, particularly hydrocarbons, on the metabolism of tubercle bacilli. Arch. Biochem. 22(1949)195-203.
21. SZEJTLI, J. Cyclodextrins and Their Inclusion Complexes.Budapest: Academy Editions, 1988.
22. SZEJTLI, J. Cyclodextrin Technology.Dordrecht: Kluwer Academic Publ., 1988.
23. SZEJTLI, J. The cyclodextrins and their applications in biotechnology. Carbodhydr. Polymers 12(1990)375-392.
24. SZENTE, L., SZEJTLI, J. and KATO, L. Solubilization of fatty acids and similar lipids by methylated cyclodextrins. International Cyclo-dextrin Symposium, 22-25 April 1992, Chicago, U.S.A., Minutes. Hedges, A. R., ed. Paris: Editions dc Sante, 1992, pp. 340-344.
25. UEKAMA, K., IRIE, T., SUNADA, M., OTAGIRI, M., IWASAKI, K., OKANO, Y., MIYATA, T. and KASE, Y. EfTccts of cyclodextrins on chlorpromazine induced hemolysis and central nervous system responses. J. Pharm. Pharmacol. 33(1981)707-714.
26. WHEELER, P. R., BLUMER, K. and RATLEDGE, C. Enzymes for biosynthesis de novo and elongation of fatty acids in mycobacteria grown in host cells: is Mycobacterium leprae competent in fatty acid biosynthesis? J. Gen. Microbiol. 36(1990)211 -217.
27. WHEELER, P. R., BLUMER, K. and RATLEDGE, C, Fatty acid oxidation and the β -oxidation complex in M. leprae and two axonically cultivable mycobacteria that are pathogens. J. Gen. Microbiol. 137(1991)885-893.
28. WHEELER, P. R. and RATLEDGE, C. Use of carbon sources for lipid biosynthesis in M. leprae : a comparison with other pathogenic mycobacteria. J. Gen. Microbiol. 133(1988)2111-2112.
1. Director of Research, Catherine Booth Hospital, 4375 Montelair Avenue, Montreal, Canada H4B 2J5.
2. Ph.D., Cyclolab R.D., Budapest, Hungary 1026.
3. Ph.D., Cyclolab R.D., Budapest, Hungary 1026.
Received for publication on 28 June 1993.
Accepted for publication on 30 November 1993.