- Volume 62 , Number 1
- Page: 89–98
Electron microscopic observations of the relationship between peripheral nerve tissue proper and an endoneurial capillary in a dermal lesion of a relapsed lepromatous patient
ABSTRACT
The origin of relapse in clinically cured leprosy patients and the dissemination of Mycobacterium leprae in such patients are hitherto little understood phenomena. A detailed electron microscopical examination of a small dermal nerve in a lepromatous lesion of a presently relapsed patient was carried out. Our observations showed the presence of M. leprae in Schwann cells, perineurial cells, vacuolar spaces located in axoplasm and elsewhere. The course of a capillary, entering the endoneurium f rom the epincurium via the perineurium, suggested the possibility of hematogenous spread of M. leprae which had been tucked away in the dermal peripheral nerve. At the time of relapse, bacilli may multiply in the nerve, may enter the bloodstream, and thus disseminate f rom the nerve into other nerves and other tissues via hematogenous spread.RÉSUMÉ
L'origine de la rechute chez des patients lépreux cliniquement guéris et la dissémination de Mycobacterium leprae chez de tels patients sont jusqu'à présent des phénomènes mal compris. On a réalisé un examen détaillé au microscope électronique d'un petit nerf dermique dans une lésion lepromatcuse d'un patient présentant une rechute. Nos observations ont montré la présence de M. leprae dans des cellules de Schwann, des cellules peri-neurales, des espaces vacuolaires localisés dans l'axoplasmc et ailleurs. Le parcours d'un capillaire, entrant dans l'endonèvre à partire de l'épinèvre via le périnèvre suggéra la possibilité d'une dissémination hématogène de M. leprae qui ont été rejetés dans le nerf dermique périphérique. Au moment de la rechute, des bacilles peuvent se multiplier dans le nerf, peuvent pénétrer dans le flux sanguin, et donc se propager à partir du nerf dans d'autres nerfs et d'autres tissus via une dissémination hématogène.RESUMEN
El orígen de las recaídas en los pacientes con lepra clinicamente curada, y Ia diseminación de M. leprae en tales pacientes, son fenómenos actualmente poco entendidos. El examen detallado por microscopia electrónica de un pequeno nervio dérmico en una lesión lepromatosa de un paciente con lepra recurrente, mostro la presencia de M. leprae en las células de Schwann, en las células perineuriales, en los espacios vacuolares localizados en el axoplasma, y en otros sítios. El curso de un capilar penetrando dei epineurio al endoneurio, via el perincurio, sugirió la posibilidad de la dispersion hematógena del M. leprae previamente enclaustrado en los nervios periféricos. Hacia el tiempo de la recaída, los bacilos se pueden multiplicar en el nervio, pueden entrara la corriente sanguinea y asi pueden diseminarse a otros nervios y a otros tejidos, via su dispersion hematôgena.An affinity and predilection of Mycobacterium leprae for peripheral nerves is seen in all forms and stages of leprosy, a disease characterized by peripheral nerve lesions. The invasion and colonization of leprosy bacilli in axons or Schwann cells is unique among bacterial cells. It is even said that M. leprae is the only bacterium penetrating the endoneurium (23). Pathological changes of leprosy nerve lesions have been studied and described by many workers using the light microscope (5, 9, 17, 21, 22, 25-27) and also the electron microscope (1-8, 10, 11, 13, 15, 18, 19, 24-27).
In several studies of peripheral nerves in the past, especially at the level of the electron microscope, most of the research workers on leprosy have limited their attention to nerve tissue of the named larger peripheral nerves. There are only a few ultrastruc- tural observations of dermal nerves (8, 13). The ultrastructural features of a small peripheral nerve in the dermal lesion of a relapsed lepromatous patient and the interconnection between the peripheral nerve and a capillary running adjacent to the nerve in the dermis were studied at the electron microscopic level by means of serial semithin sectioning. The results are reported here.
MATERIALS AND METHODS
Clinical features and bacteriology of a relapsed leprosy patient
The clinical features were as follows: a) loss of eyebrows and eyelashes; b) numerous ill-defined macules on the skin; c) lepromatous appearance of lesions on the cheek; d) loss of sensation in the lesions; and e) cord-like enlargement of the great auricular nerve. The Mitsuda test was negative. The bacterial index (BI) was 6+ by Ridley's criteria. So-called short forms of the bacilli were detected under the light microscope.
The patient had been treated with oral dapsone monotherapy for approximately 5 years. As he relapsed, the treatment with antileprosy drugs was started after biopsy as follows: clofazimine 100 mg once a day on Sunday; rifampin 150 mg once on Monday, Wednesday and Friday; ofloxacin 100 mg three times a day on Tuesday, Thursday and Saturday. This administration trial is to be continued until the patient becomes smear negative.
Tissue
A skin biopsy from a macular lepromatous lesion on the left lumbar region was excised before treatment. The biopsy was cut in half; one half was used for light microscopy: this biopsy was fixed in 10% Formalin for either routine staining by hematoxylin and eosin or Ziehl-Neelsen for the examination of bacilli in histological sections; the other half was used for electron microscopy: fixation, dehydration, and embedding of the materials were carried out as described previously (12).
RESULTS
Light microscopy
In paraffin sections, there were focal concentrations of inflammatory cells centered around dermal blood capillaries, and around fine peripheral nerves and hair follicles. After Ziehl-Neelsen staining AFB were observed, especially in blood capillaries and fine nerves, but they were relatively short.
Electron microscopy
Longitudinal sections of the peripheral dermal nerve. Photomicrographs of 300 serial, semithin longitudinal sections of the peripheral dermal nerve were made with the electron microscope (JEOL 1200EX) and examined in detail. Thirty photomicrographs containing a peripheral nerve were selected for this paper, and they are displayed in Figure 2. One of the sections (Figs. 2-4, 227) has been enlarged and is demonstrated in Figure 1. In the endoneurium of this nerve, a few bacilli were observed in the axons (Fig. 1). Other bacilli were seen in the Schwann cells, endoncurial macrophages, perineurial cells and other organelles. As shown in Inset IX of Figure 1, one small blood capillary was situated in the endoneurium. The course this capillary took inside and outside of the nerve was traced through the neighboring serial sections.
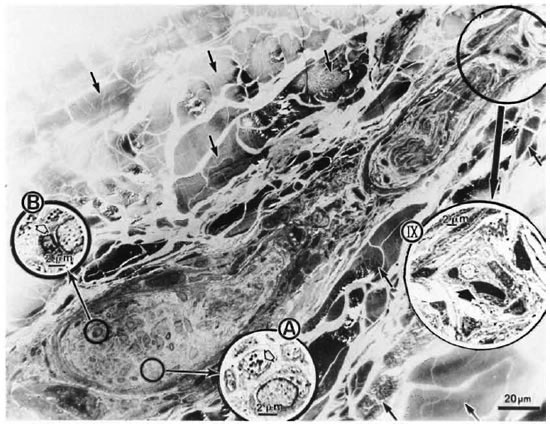
Fig. 1. Longitudinal semithin section of a small peripheral nerve, located in the collagen fiber plexus  , which is one of a total of 300 serial sections. IX = one small capillary
, which is one of a total of 300 serial sections. IX = one small capillary 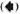 situated in the endoneurium: A and B = a few bacillary cells
situated in the endoneurium: A and B = a few bacillary cells 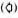 observed in vacuolar spaces located in axoplasm and seen in cross section.
observed in vacuolar spaces located in axoplasm and seen in cross section.

Fig. 2. Thirty photomicrographs were selected from the photographs of 300 serial semithin sections of the peripheral dermal nerve. 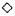 (A, B, C, etc.) and
(A, B, C, etc.) and  (a, b, c, etc.) show the cross-sections of two capillaries in the epineurium running along the perineurium;
(a, b, c, etc.) show the cross-sections of two capillaries in the epineurium running along the perineurium;  and
and indicate that a third capillary makes a turn in the direction of the endoneurium and show the course of it.
indicate that a third capillary makes a turn in the direction of the endoneurium and show the course of it.
Fig. 2-1. In the serial semithin sections it can he seen that the nerve is first cut in transverse section. The nerve then gradually turns to be cut obliquely and finally longitudinally (see also Figs. 2-2, 2-3, and 2-4).
Electron micrographs of the capillary showed that the vessel, located in the epineurium, made a turn in the direction of the endoneurium (starting in Photo 170 in Fig. 2-2 and ending in Photo 227 in Fig. 2-4). At the same location, starting at Photo 189 in Fig. 2-2 and ending at Photo 220 in Fig. 2-3, this capillary penetrated the perineurium obliquely and entered the endoneurium of the peripheral nerve (Photo 200 in Fig. 2-3 indicates the luminal caveolae in the capillary). In contrast to this capillary, two other capillaries in the epineurium and running along the perineurium (Fig. 2-2 to Fig. 2-5) seemed to follow a straight path and did not penetrate via the perineurium into the endoneurium (Photos 170-C, 220-I, 236-L and 267-Q).
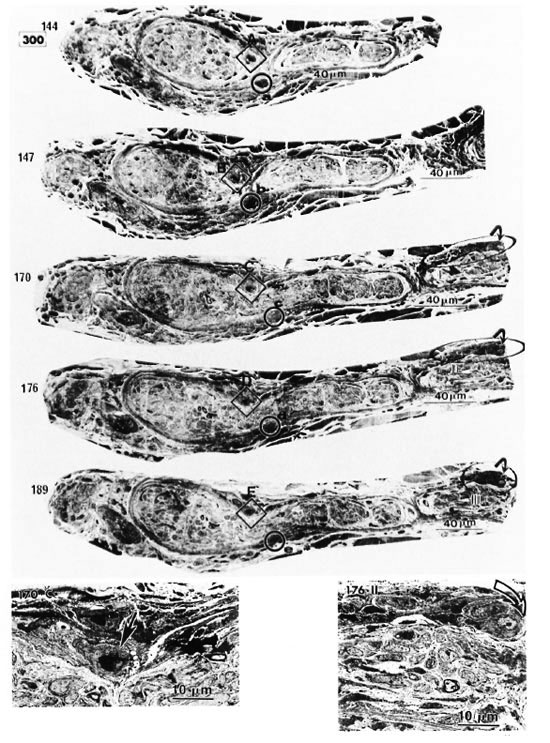
Fig. 2-2. Inset 170C is a high magnification of C in 170 (  = capillary in the perineurium); 176-II points up
= capillary in the perineurium); 176-II points up  in 176 (
in 176 ( = capillary starting a turn in direction (lithe endoneurium).
= capillary starting a turn in direction (lithe endoneurium).
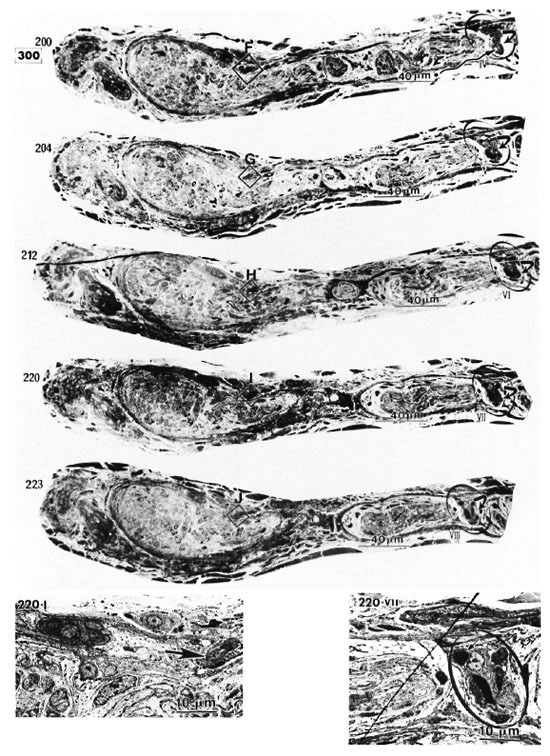
Fig. 2-3. 220-I is a high magnification of I in 220 ( = capillary).
= capillary).  in 220 VII (high magnification of
in 220 VII (high magnification of  in 220) pinpoints the capillary observed inside the endoneurium (
in 220) pinpoints the capillary observed inside the endoneurium ( = erythrocyte).
= erythrocyte).
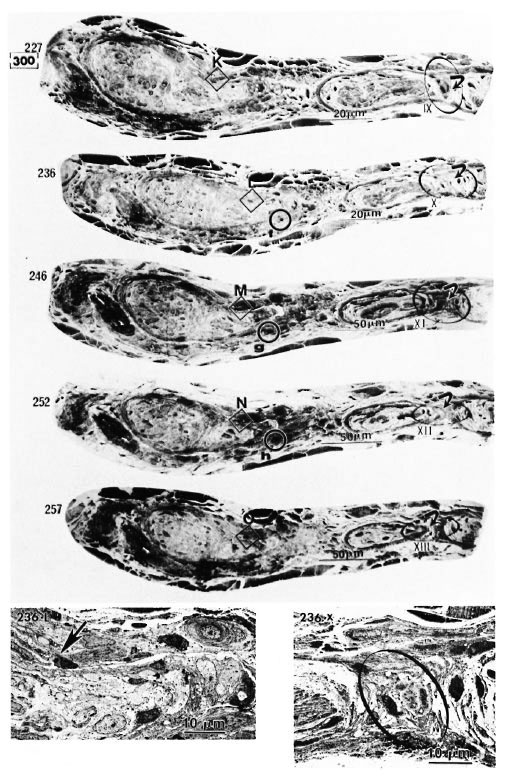
Fig. 2-4. 236-L is a high magnification of L in 236 ( = capillary).
= capillary).  in 236-X (high magnification of
in 236-X (high magnification of 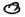 in 236) demonstrates the capillary observed inside the endoneurium.
in 236) demonstrates the capillary observed inside the endoneurium.
Fig. 2-5. 267.Q is a high magnification of Q in 267 (note in high magnification of  in 267
in 267  = capillary). 267 XV (
= capillary). 267 XV ( in 267) implies adluntinal vacuoles in the endothelium and erythrocyte-like structures
in 267) implies adluntinal vacuoles in the endothelium and erythrocyte-like structures  in the fine capillary.
in the fine capillary.
Bacilli in vacuolar spaces located in axoplasm. The electron microscopic features of the bacilli observed in vacuolar spaces located in axoplasm were diverse (Fig. 3). Some organisms were caught in the act of division. The new cell wall was clearly seen (Fig. 3a). The intracellular membranous organelles and nuclear regions of intact bacilli were well preserved (Fig. 3b and c).
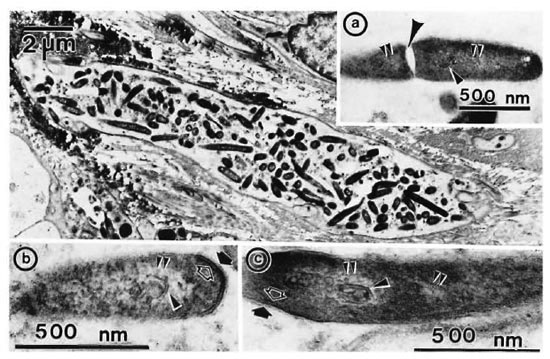
Fig. 3. Diverse forms of bacilli observed in vacuolar spaces located in axoplasm. a = Cell division is clearly caught.
is clearly caught. = cell wall:
= cell wall: 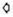 = cytoplasmic membrane;
= cytoplasmic membrane;  = nuclear region;
= nuclear region;  = intracellular membranous organelle.
= intracellular membranous organelle.
DISCUSSION
Vasa nervorum supplying peripheral nerves, in general, are derived from a series of branches from associated regional arteries, and branches from those arteries enter the epineurium to form an intercommunication or anastomosing plexus. From that plexus, vessels penetrate the perineurium obliquely and enter the endoneurium as capillaries (20). The "blood-nerve barrier" formed by closely adhering endothelial cells and constructed between the lumen of the endoneurial capillaries and the endoneurial tissue proper results in a reduced permeability which is the property of blood capillaries of nerve tissue in general (4, 16). On the other hand, it is surmised that an exchange between the exterior and interior of the intraneural vessel can take place in fenestrate capillaries. Thus, capillaries having structural variations permit different levels of metabolic exchange between blood and the surrounding tissue (4, 15).
According to Olsson and Reese (19), healthy endoneurial blood vessels act as a barrier, preventing introduced tracer molecules from entering the endoneurium, while there is a breakdown of the barrier following nerve injury. Boddingius (4) pointed out that the occurrence of very early vessel changes in named peripheral nerves in the case of leprous neuropathy may cause extensive leakage of cndoneurial blood vessels and subsequent damage to the nerve, even before signs of M. leprae were seen locally in the nerve. Thus, it was suggested that angiopathy of endoneurial vessels contributed a great deal to the degenerative changes seen in named peripheral nerves of leprosy patients. More recently, Chandi and Chacko (8), for dermal peripheral nerves, reasoned as follows: if infection of nerves takes place exclusively in an ascending manner from their peripheral terminations, it is more likely that the resultant cellular infiltrate would be derived from damaged endoneurial blood vessels rather than from across a more distant and better preserved perineurial barrier.
Quite a bit of the leprosy literature deals with the dissemination of M. leprae to other nerves and tissues (1, 2, 4,6, 8, 10, 11, 21, 25-27). Boddingius (2, 5), in particular, emphasized the early spread of leprosy bacilli via intra-axonal retrograde transport. It has been suggested in almost all of these reports that a more widespread dissemination of the bacilli would be through the bloodstream, especially via the endoneurial capillaries. However, such an hypothesis of a hematogenous route is only a view deduced from observations on single sections, and thereis no substantive evidence.
In order to understand the architecture of tissue cells or compounds in biomaterials, it is necessary to study sections made in different planes by electron microscopy and to reason accordingly. Low-magnification electron micrography of serial sections, in particular, is fundamental to a true comprehension of a limited part of a biopsy (12). So far no paper has appeared to show the microcytomorphological analysis of a peripheral nerve in the dermis of a lepromatous lesion, based on low-magnification electron micrography using serial sections. In the present study this micrography was used to elucidate the architecture of the interconnection between a peripheral nerve and a capillary running adjacent to the nerve in the dermis.
In this study, the course of a capillary penetrating from the epineurium into the endoneurium, or vice versa, was observed in the dermal nerve in a lepromatous lesion, (Fig. 2). A few bacillary cells were demonstrated in vacuolar spaces located in axoplasm of the same nerve (Fig. 1A and B, Fig. 3).
In consideration of these facts, it may be surmised that hematogenous spread of the bacilli could occur by bacilli breaking out of vacuolar spaces located in the axoplasm or axons and then entering the endoneurial capillary in lepromatous leprosy. That is, bacilli having entered the nerve once and proliferated in axonal tissues and in Schwann cells are possibly moving from the nerve tissue into the bloodstream. This would mean there is a definite defect of the bloodnerve barrier in the endoneurial capillary. It has been confirmed by many workers (1-4, 8, 11, 13, 14, 26) that bacillary invasion of axons occurs in the case of lepromatous leprosy. Most likely, intra-axonal organelles and/or molecular substances are conveyed in retrograde direction by axoplasmic transport (20). Therefore, it is possible that the widespread dissemination of leprosy bacilli, particularly in the case of relapsed leprosy, is first through intra-axonal transport and then through penetration of the small openings in the endothelium of capillaries in the endoneurium, leading further to extensive hematogenous spread.
The foregoing considerations may be summed up briefly as follows: bacilli having settled in the endoneurium and remaining inactive for a long period are eventually discharged into the lumen of parasitized vessels when there is a favorable opportunity. The present study dealt only with one case of relapsed leprosy and the results cannot be considered definitive. Further submicroscopic exploration of the locations of M. leprae in dermal nerves or named peripheral nerves in untreated or relapsed leprosy is needed in order to understand fully the precise nature of the connection between the peripheral nerve tissue proper and the endoneurial capillary.
REFERENCES
1. BODDINOIUS, J. The occurrence of Mycobacterium leprae within axons of peripheral nerves. Acta Neuropath. (Berl.) 27(1974)257-270.
2. BODDINGIUS, J. Ultrastructural changes in blood vessels of peripheral nerves in leprosy neuropathy. I. Tuberculoid and borderline-tuberculoid leprosy patients. Acta Neuropath. (Berl.) 35(1976)159-181.
3. BODDINGIUS, J. Ultrastructural changes in blood vessels of peripheral-lepromatous and lepromatous leprosy patients. Acta Neuropath. (Berl.) 40(1977)21-39.
4. BODDINGIUS, J. Ultrastructural and histophysiological studies on the blood-nerve barrier and perineurial barrier in leprosy neuropathy. Acta Neuropath. (Berl.) 64(1984)282-296.
5. BODDINGIUS, J., REES, R. J. W. and WEDDELL, A. G. M. Defects in the blood-nerve barrier in mice with leprosy neuropathy. Nature (New Biol.) 237(1972)190-191.
6. BURCHARD, G.-D. and BIERTHER, M. An electron microscopic study of small cutaneous vessels in lepromatous leprosy. Int. J. Lepr. 53(1985)70-74.
7. CHANDI, S. M and CHACKO, C. J. G. An ultrastructural study of the response of traumatized rabbit tibial nerve to epincurial infection with Mycobacterium leprae . Int. J. Lepr. 54(1986)79-83.
8. CHANDI, S. M. and CHACKO, C. J. G. An ultrastructural study of dermal nerves in early human leprosy. Int. J. Lepr. 55(1987)515-520.
9. CORUH, G. and MCDOUGALL, A. C. Untreated lepromatous leprosy: histopathologic findings in cutaneous blood vessels. Int. J. Lepr. 47(1979)500-511.
10. DASTUR, D. K., RAMAMOHAN, Y. and SHAH, J. S. Ultrastructure of lepromatous nerves; neural pathogenesis in leprosy. Int. J. Lepr. 41(1973)47-80.
11. FINLAYSON, M. H., BILBAO, J. M. and LOUGH, J. O. The pathogenesis of the neuropathy in dimorphous leprosy: electron microscopic and cytochemical studies. J. Neuropath. Exp. Neurol. 33(1974)446-455.
12. HIRATA, T. Low-magnification electron micrography of leproma in human skin based on semithin and ultra thin sectioning. Lepr. Rev. 61(1990)227-236.
13. IMAEDA, T. and CONVIT, J. Electron microscopic study of cutaneous nerves in leprosy. Int. J. Lepr. 31(1963)188-210.
14. JOB, C. K.and VERGHESE, R. Electronmicroscopic demonstration of Myco. leprae in axons. Lepr. Rev. 45(1974)235-239.
15. JOB, C. K., VICTOR, D. B. I. and CHACKO, C. J. G. Progressive nerve lesion in a disease-arrested leprosy patient; an electron microscopic study. Int. J. Lepr. 45(1977)255-260.
16. JUNQUEIRA, L. C, CARNEIRO, J. and KELLEY, R. O. Basic Histology. 7th edn. Englewood Cliffs, NJ : Prentice-Hall International Inc., 1992.
17. KHANOLKAR, V. R. Pathology of leprosy. In: Leprosy in Theory and Practice. Cochrane, R. G. and Davey, T. F., eds. Bristol: J. Wright & Sons Ltd., 1964, pp. 125-151.
18. NISHIURA, M., HARADA, N. and IMAEDA, T. Electron microscopy of ultrathin sections of lepromatous peripheral nerves. Int. J. Lepr. 25(1957)323-328.
19. OLSSON, Y. and REESE, T. S. Permeability of vasa nervorum and perineurium in mouse sciatic nerve studied by fluorescence and electron microscopy. J. Neuropath. Exp. Neurol. 30(1971)105-1 19.
20. ORITIZ-HIDALGO, C. and WELLER, R. O. Peripheral nervous system. In: Histology for Pathologists. Sternberg, S. S., ed. New York: Raven Press Ltd.,1992, pp. 169-193.
21. PEARSON, J. M. H. and Ross, W. F. Nerve involvement in leprosy-pathology, differential diagnosis and principles of management. Lepr. Rev. 46(1975)199-212.
22. PEARSON, J. M. II. and WEDDELL, A. G. M. Peripheral changes in untreated leprosy. Lepr. Rev. 46(1975)51-67.
23. TURK, J. L., CURTIS, J. and DE BLAQUIERE, G. Immunopathology of nerve involvement in leprosy. Lepr. Rev. 64(1993)1-6.
24. TURKEL, S. B., VAN HALE, H. M. and REA, T. H. Ultrastructure of the dermal micro vasculature in leprosy. Int. J. Lepr. 50(1982)164-171.
25. VAIDYA, M. C, PALMER, E., WEDDELL, G. and REES, R. J. W. A note on the presence of Mycobacterium leprae in the central nervous system of a mouse with lepromatous leprosy. J. Med. Microbiol. 3(1970)194-196.
26. YOSHIZUMI, M. O. and ASBURY, A. K. Intra-axonal bacilli in lepromatous leprosy; a light and electron microscopic study. Acta Neuropathol. (Berl.) 27(1974)1-10.
27. YOSHIZUMI, M. O., KIRCHHEIMER, W. F. and ASBURY, A. K. A light and electron microscopic study of peripheral nerves in an armadillo with disseminated leprosy. Int. J. Lepr. 42(1974)251-259.
1. Ph.D., Chief, Research and Training Section and Electron Microscopy Laboratory, National Institute for Leprosy Research, 4-2-1 Aoba-cho, Higashimurayama-shi, Tokyo 189, Japan.
2. Ph.D., Honorary Director, Leprosarium Oku Komyo-En, Okayama, Japan.
Reprint requests to Dr. Hirata at his present address: Raj-Pracha-Samasai Institute, Phra-Pradaeng, Samutprakarn 10130, Thailand.
Received for publication on 7 May 1993.
Accepted for publication in revised form on 25 October 1993.