- Volume 62 , Number 1
- Page: 143–7
Active humoral immunity in the absence of cell-mediated immunity in murine leprosy: lastly an explanation
To the Editor:
Until recently, a puzzling phenomenon in (human) lepromatous leprosy was that related to the patients' immune responsiveness to antigens of Mycobacterium leprae . It is a well-accepted fact that lepromatous patients show an absence of cell-mediated immunity (CMI) to M. leprae antigens while they retain unaltered their humoral (antibody-mediated) immunity (AbMI). This was puzzling, because the great majority of the microbial antigens belong to the so-called "thymus dependent" type, i.e., they need the participation of T-helper lymphocytes (LcTh) to generate efficient antibody responses. How, then, would the dramatic alteration in the T-cell-mediated immune competence of the host not reflect on its humoral competence?
An analogous situation of a gradual loss of CMI with an apparently unaltered AbMI has been found in mice suffering from "murine leprosy," a disease caused by M. lepraemurium (MLM) and characterized by the development of granulomatous lesions in the skin and viscera that highly resemble the lepromatous lesions of human leprosy.
In both mycobacterioses, several investigations point to a deficit in the function of the helper population of T lymphocytes due to the absence of antigen-reactive T cells, the lack of interleukin-2 (IL-2)-producing cells, the excess of suppression by suppressor T(CD8+ or Ly2,3+)cells, the suppression by macrophage-derived factors, etc. (for a recent review on the subject, see 11). Those steps within the intricate net of cellular interactions that have been found altered in human lepromatous or mouse lepromatoid leprosy and that could, to a certain extent, explain the finding in both diseases of depressed (or absent) CMI to the mycobacterial antigens, are illustrated in an oversimplified manner in Figure 1.
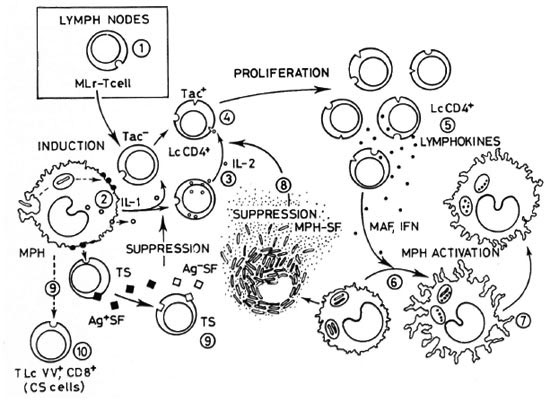
Fig. 1. Cell interactions in cell-mediated immunity, showing those steps that have been found altered (or normal in contradictory reports) in lepromatous leprosy.  availability of circulating M. leprae -reactive (MLr) T cells; some authors suggest that a great amount of lymphocytes are trapped into the lymph nodes, leading to the lowering of their levels in circulation;
availability of circulating M. leprae -reactive (MLr) T cells; some authors suggest that a great amount of lymphocytes are trapped into the lymph nodes, leading to the lowering of their levels in circulation; antigen handling and/or interleukin-1 (IL-1) production by macrophages (MPH),
antigen handling and/or interleukin-1 (IL-1) production by macrophages (MPH),  synthesis and release of IL-2 by MLr T cells;
synthesis and release of IL-2 by MLr T cells;  expression of IL-2 receptors (Tac) by Thelper/inducer cells;
expression of IL-2 receptors (Tac) by Thelper/inducer cells;  IL-2/antigen-driven proliferation of T cells with concomitant synthesis and release oflymphokines (depending on steps I to 4);
IL-2/antigen-driven proliferation of T cells with concomitant synthesis and release oflymphokines (depending on steps I to 4);  bactericidal response of macrophages to the infecting mycobacteria;
bactericidal response of macrophages to the infecting mycobacteria;  lymphokine-dependent activation of macrophage;
lymphokine-dependent activation of macrophage; 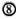 suppressive effects of mycobacterial components andfactors released from bacilli-laden macrophages,
suppressive effects of mycobacterial components andfactors released from bacilli-laden macrophages,  ML-induced activation of CD8+ T (suppressor) cells (TS)able to produce antigen-specific suppressor factors (Ag + SF), and Ag + SF-dependent activation of third-party lymphocytes able to produce nonspecific suppressor factors (Ag-SF);
ML-induced activation of CD8+ T (suppressor) cells (TS)able to produce antigen-specific suppressor factors (Ag + SF), and Ag + SF-dependent activation of third-party lymphocytes able to produce nonspecific suppressor factors (Ag-SF);  downregulation by Vicia villosa ("contrasuppressor") cells (CS) (see 11 for a more detailed description of this figure).
downregulation by Vicia villosa ("contrasuppressor") cells (CS) (see 11 for a more detailed description of this figure).
For a long time, in the mouse as well as in humans the existence and function of only one class of helper (L3T4 + /CD4 + ) T lymphocyte (a Th lymphocyte able to mediate cellular immunity and, at the same time, able to cooperate with B lymphocytes for antibody production) was accepted (Fig. 2). The existence of a "bifunctional" T cell, however, does not help to explain the persistence of an active humoral immunity in the absence of a helper CMI to the mycobacterial (thymus-dependent) antigens.
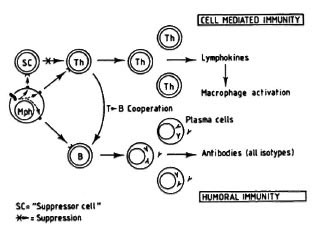
Fig. 2. A T lymphocyte acts as a bifunctional cell, both directly responsible for CMI and also able to cooperate with B cells for antibody production (all isotypes included). Here, the main regulatory mechanism is exerted by a suppressor cell, either a lymphocyte, a macrophage, or some other cell. The absence of specific T cells would lead to the lack of a CMI response to a given antigen and to a parallel fall in antibody response to that antigen.
On the other hand, the still recent discovery of two subpopulations of helper (L3T4+) T cells in the mouse (Th1 and Th2) (2,3,9,10) allows one to tentatively explain the apparent paradox mentioned above. Both Th 1 and Th2 clones produce and release IL-3, GM-CSF, TNF-a, in addition to other proteins. However, while TH1 clones secrete IL-2, interferon- γ and lymphotoxin,Th2 clones secrete IL-4, IL-5, IL-6, and IL-10. It has been proposed that although Th1 cells may cooperate with B cells, their main roll is related to lymphokine production, macrophage activation and delayed hypersensitivity (2). The Th2 clones, on the other hand, are more cooperative with B cells (14). Th1 cells and Th2 cells seem to be mutually inhibitory (Th1-derived IFN- γ inhibits the in vitro proliferation of Th2, and Th2-derived IL-4 or IL-10 might inhibit the proliferation of Th1 clones (7). Since in advanced murine leprosy CMI to MLM antigens is absent but humoral immunity is not, it is possible that Th2 cells may be the predominant (at least functionally) subpopulation. Thus, contrary to Figure 2, Figure 3 takes into consideration the participation of two subclasses of T helper lymphocytes and explains, reasonably well, the depressed or absent cellular immunity in the presence of a hyperactive antibody-mediated immunity.
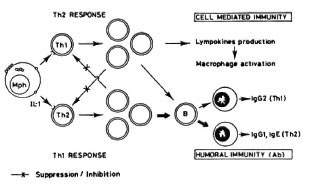
Fig. 3. Involvement of two T cells (with the helper/inducer phenotype), one engaged in CMI, the other able to potentiate the activity of B cells, and both mutually inhibitory, explains reasonably well the situation observed in leprosy of deficient or absent CMI to the mycobacterial antigens in the presence of normal ability to produce antibodies to the same antigens.
This is the situation reported repeatedly in leprosy. On activation (in an Ia-restricted manner), Thl lymphocytes produce IL-2 and respond to it, to eventually proliferate and give rise to the elements of CMI and delayed hypersensitivity. It is through soluble factors (such as IFN- γ ) that Thl lymphocytes activate macrophages increasing their microbicidal capabilities (l4). An excess of 1FN- γ may, however, suppress the function of Th2 lymphocytes (6). Activated Thl lymphocytes seem to cooperate with B cells to induce the synthesis of IgM, IgG3 and IgG2. Activated Th2 cells, to the contrary, produce among other factors IL-4,IL-5 and IL-10. IL-4 will suppress the ac-tivation of Thl cells at the same time thatit promotes differentiation and activation of B cells. B cells activated through Th2 cells produce IgM, IgG3, IgG1 and IgE (13). B cells and macrophages function as APC forboth Thl and Th2 cells. Although able tocooperate with B cells, Th 1 cells are less cooperative than Th2 cells (8).
Since in lepromatous (human and murine) leprosy, there is a gradual loss of CMI that is inversely proportional to the degree of antibody response to the mycobacterial antigens, it would seem that initially Thl cells are the predominantly active T lymphocytes, while the predominant cell population in the advanced disease are the Th2 lymphocytes.
Although the mycobacterial epitopes that stimulate each T-cell subpopulation remain to be identified, it would seem that the immunodominant epitopes (both in human lepromatous and murine leprosy) are those that trigger the Th2 (or human equivalent) (1) cells, rather than those that trigger the Th1 (or human counterpart) (1) cells. Accordingly, other epitopes would preferentially activate the Th1 cells in tuberculoid leprosy (if there is any) in the mouse. We have demonstrated that early in the infection with M. lepraemurium , the granulomatous lesions are made up of biochemically activated macrophages intermixed with lymphocytes (a tuberculoid-type granuloma). Then, in the late stages of the disease, the macrophages' biochemical activation subsides and the lesion acquires the characteristics of lepromatous granulomas (inertand highly bacilliferous macrophages) (12).Thus, it is possible that activation of Th1 and Th2 lymphocytes (or the human equivalents) may occur in sequence but, so far, neither the events that drive such a sequence nor the underlying regulatory mech-anisms are well understood.
Additionally, other factors more than mere mycobacterial epitopes might also participate in the differential triggering of each subpopulation of L3T4+ cells: inadequate participation of APC, whether a macrophage or a B lymphocyte (see below); diverse factors (cytokines) might also tilt the balance in favor of either the Th1 or the Th2 subpopulation (5); some genetic factors (not necessarily MHC-related) might also participate in the turning on/off of the Thl or Th2 cells, etc. At the moment, all of these factors are ill-defined and, hence, they are difficult to evaluate in vivo . The use of monoclonal antibodies to several known interleukins would permit the identification and measurement of the IL-2 and IL-6 (or IL-4) levels in the serum of infected individuals and the localization of IL-2-producing (Th1) and IL-6-producing (Th2) lymphocytes in the lesions, so their participation in the immunopathology of the disease might possibly be assessed. Alternatively, Thl and Th2 cells in the lesions also could be identified by Northern hybridization of poly(A) + mRNA isolated from the spleen and lymph nodes at variable times after infection with M. lepraemurium with probes coding for IL-4, IL-6, IL-10, IL-2,and IFN- γ , in the way that has been done for mice infected with Leishmania major (7), or by the more practical and sensitive polymerase chain reaction (PCR) with probes for the above lymphokines. Also, although both Th1 and Th2 cells might be able to induce secretion of IgM and IgG3 immunoglobulins, IgGl (and IgE) secretion seems to be induced solely by Th2 cells and IgG2a solely by Thl cells (4). Serological identification and measurement of these immunoglobulin isotypes would allow one to decide which L3T4+ is the dominant participant at a given stage of the disease.
The regulation of the immune response by the participation of B lymphocytes as APC is a possibility that deserves further consideration since these cells can efficiently present antigen to T cells without producing significant amounts of IL-1. This means that B cells functioning as APC would activate mainly or solely those Th cells that are IL1-independent (Thl cells). On activation, these cells would produce INF- γ which, in turn, might suppress the function of the Th2 subpopulation (6), leading to the overall lowering of the antibody response. On the other hand, a defective or a short-lived APC function of B cells would not efficiently promote activation of lymphocytes, whether or not IL-1-dependent. The APC function instead will be exerted by the constitutively IL-1 -producing macrophages. Activation of T cells through macrophages will then favor activation of the Th2 subpopulation, with the consequent increase in antibody response and the simultaneous inhibition of the Thl cell subpopulation. Thus, it is proposed that in those animals that develop tuberculoid-like disease, the antigen-presenting macrophages initiate the response, allowing the activation of both IL-1-dependent (Th2) and IL-1-independent (Th1) lymphocytes. As B cells start functioning as APC, they will favor activation of IFN- γ producing (IL-1-independent) Thl cells. IFN- γ would inhibit the function of Th2 cells, leading to the lowering of the antibody response (Fig. 4A).
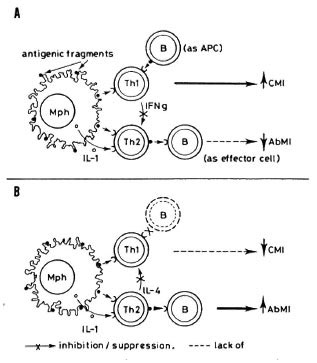
Fig. 4. A = Functional B cells (B) and macrophages(Mph), acting as antigen-presenting cells (APC) stimulate both the CMI-commited lymphocytes (Th1) and those that cooperate with B cells for increased production of antibodies (Th2). It is proposed that at anearly stage of the infection, Th I cells receive more inductive stimulation that do Th2 cells, favoring the CMI response and the suppression of Th2 cells, with the consequent lowering of the antibody-mediated response. Theoretically, B cells are not efficient APC toTh2 lymphocytes because Th2 cells require, in addition, the stimulatory effect of IL-1 and B cells do not produce significant amounts of it. B = As a consequence of the infection, B cells might deteriorate, losing their ability to function as APC. this would diminish the activity of Thl cells (decreasing CMI) without altering the capacity of Th2 cells to respond to the stimulatory effects of macrophages. Activated Th2 cells would further inhibit the functioning of Th I cells (and CMI) while retaining their potentiating effect on B lymphocytes.
In those animals with lepromatoid leprosy, B cells might not persist or even appear as APC. With this function carried out solely by macrophages, activation of IL-1-dependent Th2 cells would be the one to be supported, and their resulting products (IL-4 and IL-10) would eventually shut off activation of Thl cells; this would result in the depression of CM I and the sustained production of antibodies (Fig. 4B). At the moment, because the above proposition is merely speculative, the reasons why B cells might not appear or persist as APC are also speculative. One way to test the hypothesis, however, could be the administration of functional, virgin or educated, B cells from normal or tuberculoid animals into animals in which the presence of inefficient (or the absence of) antigen-producing B cells is suspected; this could retard or even revert the lepromatoid evolution of the disease. On the other hand, administration of lepromatoid-dcrived B cells to tuberculoid animals could perhaps drive the course of the infection toward the lepromatoid pole.
- Oscar Rojas-Espinosa, Sc.D.
Departamento de Inmunologia
Escuela Nacional de Ciencias Biologicas
Instituto Politécnico Nacional
Carpio y Plan de Ayala
Colonia Santo Tomas
11340 México, D.F., México
Acknowledgment. The author thanks Dr. L. Jiménez Zamudio for his enlightening discussions on the contents of this essay. The author holds a fellowship from the Instituto Politécnico Nacional, Mexico, from the Comisión de Operación y Fomento de las Actividades Académicas del I.P.N, and from the Sistema Nacional de Investigadores, México.
REFERENCES
1. BLOOM, B. R., MODLIN, R. L. and SALGAME, P. Stigma variations-observations on suppressor T-cells and leprosy. Ann. Rev. Immunol. 10(1992)453-488.
2. CHER, D. J. and MOSMANN, T. R. TWO types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by Thl clones. J. Immunol. 138(1987)3688-3694.
3. CHERWINSKI, H. M., SCHUMACHER, J. H., BROWN, K. D. and MOSMANN, T. R. TWO types of mouse helper T cells clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J. Exp. Med. 166(1987)1229-1244.
4. COFFMAN, R. L., SEYMOUR, B. W., LEDMAN, D. A., HIRAKI, D. D., CHRISTIANSEN, J. A., SHRADER, B., CHERWINSKI, H. M., SAVELKOUL, H. F., FINKEL-MAN, F. D., BOND, M. W. and MOSMANN, T. R. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol. Rev. 102(1988)5-28.
5. FERNANDEZ-BOTRAN, R., SANDERS, V. M., MOSMANN, T. R. and VITETTA, E. S. Lymphokine-mediated regulation of the proliferative response of clones of T helper 1 and T helper 2 cells. J. Exp. Med. 168(1988)543-558.
6. GAJEWSKI, T. F. and FITCH, F. W. Anti-prolifcrativc effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Thl murine helper T lymphocyte clones. J. Immunol. 140(1988)4245-4252.
7. HEINZEL, F. P., SADICK, M. D., HOLADAY, B. J., COFFMAN, R. L. and LOCKSLEY, R. M. Reciprocal expression of interferon gamma or interlcukin 4 during the resolution or progression of murine leishmaniasis; evidence for expansion of distinct helper T cell subsets. J. Exp. Med. 169(1989)59-72.
8. KILLAR, L., MACDONALD, G., WEST, J., WOODS, A. and BOTTOMLY, K. Cloned, Ia-restricted T cells that do not produce interleukin 4(IL-4)/B cell stimulatory factor 1 (BSF-1) fail to help antigenspecific B cells. J. Immunol. 138(1987)1674-1679.
9. MOSMANN, T. R., CHERWINSKI, H., BOND, M. W., GIEDLIN, M. A. and COFFMAN, R. L. TWO types of murine helper T cell clone. I. Definition according to profiles of lymphokinc activities and secreted proteins. J. Immunol. 136(1986)2348-2357.
10. MOSMANN, T. R. and COFFMAN, R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv. Immunol. 46(1989)111-147.
11. ROJAS-ESPINOSA, O. and ESTRADA-PARRA, S. Immunology of leprosy: cellular ancrgy to Mycobacterium leprae . Arch. Invest. Med. 20(1989)335-341.
12. ROJAS-ESPINOSA, O., VEGA, R., OLTRA, A., ARCE, A. and NUÑEZ, A. Transitory macrophage activation in the granulomatous lesions of Mycobacterium leprae murium -induced lepromatoid leprosy in the mouse. Int. J. Lepr. 56(1988)428-436.
13. STEVENS, T. L., BOSSIE, A., SANDERS, V. M., FERNANDEZ-BOTRAN, R., COFFMAN, R. L., MOSMANN, T. R. and VITETTA, E. S. Regulation of antibody isotype secretion by subsets of antigenspecific helper T cells. Nature (Lond.) 334(1988)255-258.
14. STOUT, R. D. and BOTTOMLY, K. Antigen specific activation of effector macrophages by IFN- γ -producing (Th1) T cell clones; failure of IL-4-producing (Th2) T cell clones to activate effector function in macrophages. J. Immunol. 142(1989)760-765.