- Volume 62 , Number 1
- Page: 135–9
A latex agglutination test for rapid serodiagnosis of leprosy in the field
To the Editor:
At present leprosy is diagnosed and classified according to clinical, histopathological, and bacteriological criteria (1). Nevertheless, in many developing countries where leprosy is endemic resources for these purposes are often very limited. Therefore, it is necessary to have simple, cost-effective, and rapid methodologies which can be complementary to, or even substitute for, conventional diagnostic procedures. The Mycobacterium leprae -specific phenolic glycolipid (PGL-I) in the semisynthetic disaccharide octyl bovine serum albumin (ND-O-BSA) form (5) has been widely used in indirect enzyme-linked immunosorbent assay (ELISA) techniques to detect anti-PGL-I antibodies in a number of diagnostic and epidemiologic studies (e.g.,2, 7, 11). Even though the ELISA has many advantages, being fast, reproducible and quantitative, it does need the framework of an established laboratory and trained personnel.
The objective of this work was to develop a simple latex particle agglutination assay (LPAA) that can be performed under subtropical and tropical field conditions by untrained personnel which would give an easy, visual read-out within 5 minutes and which is cost-effective.
Latex particles (Estapor, KI-080, azure; Rhone-Poulenc, Aubervilliers, France) were covalently coupled with 400 µ g/ml ND-O-BSA, obtained through WHO IMMLEP, according to the manufacturer's instructions (Rhone-Poulenc). Finally, precipitated antigen coupled latex particles were resuspended in 0.5 ml of the binding buffer (50 mM Na2P04, 17 mM NaCl, 0.025% v/v Nonidet P-40, 0.1% w/v gelatin, 2% w/v Kolhdon VA64, pH 6.6). A 5 µ l suspension was spotted onto Perlalux Duplex rigid plastic films (Papierfabrik Perlen, Perlen, Switzerland) as a dot. The dots were dried at 40ºC for 1 hr and stored at room temperature until used. Stability tests for binding to the plastic film, solubility, agglutination, and sensitivity were performed after 1 week, 2 weeks, 1 month, 2, 3, 6, 9, and 12 months using a standard pool of leprosy sera with a shelf-life of at least 12 months when stored at 15ºC-35ºC and 40%-80% humidity (data not shown). Hereby the criteria for use under subtropic and tropic conditions without refrigerating were obtained.
For the LPAA, 10 µl undiluted serum or twofold dilutions made in phosphate buffered saline, pH 7.2, of negative control serum, positive control serum and the unknown serum sample were added to the dried latex dots. After 60 sec, the dots were resuspended with a spatula and mixed gently. Four min later the results were read by visual inspection. The antibody titer was expressed as the highest dilution giving agglutination. A sample was considered positive when the titer value was 2 or more, determined on the basis of three times the standard deviation of the Ethiopian control group. The ELISA was used to test the reliability of the LPAA. The assay method was similar in principle to the one described by Cho, et al. (5). However, 2.5% w/v casein and 0.05% v/v Tween 20 were used as the blocking agent instead of 5% w/v BSA. Serum samples were tested as twofold dilutions starting at the 1:200 dilution. The ELISA plates were Maxisorp Immunoplate (Nunc A/S, Roskilde, Denmark). Peroxidase-conjugated anti-human immunoglobulin M (P217; Dako A/S, Glostrup, Denmark) was used as the detecting secondary antibody. O-Phenylenediamine (OPD) and H2O2 in citrate phosphate buffer, pH 5.0, were used as the enzyme substrate. The value of the ELISA results are given as multiples of negative activity (MONA) values. A sample was considered positive when the MONA value was 4 or more, determined on the basis of three times the standard deviation of the Ethiopian control group.
The serum samples for this study were collected from 57 leprosy patients (43 Ethiopians and 14 Danes), 26 healthy Ethiopians, 12 Danish tuberculosis patients and 23 healthy Danes. The leprosy sera were classified according to the Ridley-Jopling scale (12) as 27 multibacillary patients (1 BB, 13 BL, 13 LL) and 30 paucibacillary patients (26 BT, 4 TT). Because of the subjective estimate of analysis on the degree of agglutination, all sera were tested under code at least twice before the results were sent to the Armauer Hansen Research Institute (AHRI) where the code was opened. At AHRI the results of both the LPAA and the ELISA were compared to previously obtained results.
Spearman's correlation coefficient was used for comparison between the groups (14). In ELISA we measured the IgM antibody responses (data not shown) because previously obtained data (e.g., 15) showed only IgM responses to be of significance in the serodiagnosis of leprosy. The Spearman's correlation coefficients between the LPAA and the mean absorbance of IgM and IgG antibodies were 0.9215 and -0.3214 for the multibacillary cases, respectively.
Table 1 summarizes the results of the LPAA and ELISA. The agreement between the results obtained for the LPAA in Copenhagen and the ELISA performed previously in Addis Ababa was determined by Spearman's correlation coefficient. Values obtained included: 0.9992 for multibacillary leprosy patients, 0.9643 for multibacillary multidrug-treated patients, 0.9960 for paucibacillary patients, 1.0 for paucibacillary multidrug-treated patients, and 0.9985 for the Ethiopian control group, respectively, all showing good correlation between the data obtained by the two methods.
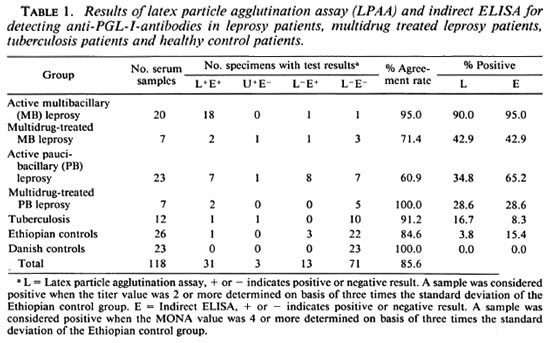
Because the aim of this study, among other things, was to develop a serodiagnostic leprosy assay to be used in the field under difficult conditions, we also tested undiluted sera in the LPAA (Table 2). Generally the sensitivity of the LPAA was higher than the results shown in Table 1, but when we consider the healthy Ethiopians as the negative control group, the specificity of the LPAA fell from 96.2% to 76.9%, compared to the result obtained in Table 1, as a result of an increased number of patients' sera having low antibody titers against PGL-I.
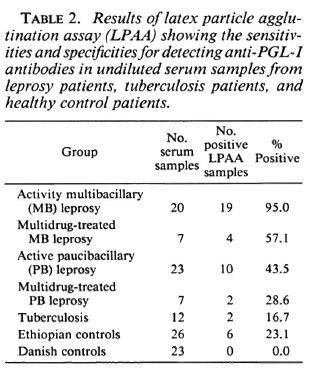
Recently, papers have been published describing the rapid serodiagnosis of leprosy using latex agglutination assays (6, 8, 14) and using a gelatin particle agglutination assay (9). Clearly, these efforts represent improvements, and may assist the clinician in the diagnosis of leprosy. However, the assay system described in the present work is even simpler to perform and is not dependent on access to any laboratory facilities. The reagents needed for the LPAA described here can be stored for 1 year at ambient temperature, and the assay may be performed using undiluted serum. The test may also prove useful in low-endemic areas where the number of patients suspected of having leprosy is so low that even an ELISA would be uneconomical to set up. We have compared the LPAA using both an undiluted and a diluted series of sera. The use of undiluted sera has a practical advantage and, in fact, increases the sensitivity of the system. When we prepared a twofold dilution series of the sera, the LPAA was found to be more specific than the indirect ELISA using the Ethiopian control group as a reference. The false-positive reactions (4 out of 26) observed in the Ethiopian control population may be caused by the relative hyper-gammaglobulinemia which is more common among Ethiopians compared to Scandinavians (10). It should also be kept in mind that the Ethiopian population was unselected and might have included healthy contacts or subclinical cases who had developed anti-PGL-I antibodies without any sign of the disease.
Using the dilution series of the sera we found that sensitivity was 90.0% for the LPAA when considering multibacillary patients, which is comparable with the other described assays 95.2% (3), 62.7% (8), 79.7% (9), and 88.4% (14). The paucibacillary patients generally displayed a very low level of anti-PGL-I antibodies; we detected 34.8% by the LPAA. Considering this, our test is as effective for the paucibacillary sera as the gelatin particle agglutination test described previously (3, 9). They obtained sensitivities of 21% and 33.3%, respectively, with specificities comparable to results obtained in this study. Wu, et al. (14) described a sensitivity at 62.5% for paucibacillary patients.
There have been many reports of decreases in antibody titer following effective chemotherapy (e.g., 4, 5). The results of the LPAA presented here agree with those of earlier reports, indicating that the LPAA may be used to monitor anti-PGL-I titers in multibacillary patients under multidrug treatment. The LPAA results can (because the latex agglutination dot dries up on the plastic sheet) be stored in the patient's file for later evaluation of the treatment.
In conclusion, the evidence presented in this communication shows that the LPAA: a) has sensitivity and specificity that can be compared with ELISA, b) may be a useful tool in the diagnosis and monitoring of multibacillary leprosy, c) can be performed with undiluted sera, d) is very easy to apply by untrained personnel under difficult field conditions, 0 the LPAA plates can be stored under subtropical and tropical conditions without refrigeration for at least 1 year without losing sensitivity, g) is rapid and gives a clear, visual read-out within 5 min because of the use of azure latex particles on a white background, h) the LPAA plates can, after assay, be stored in the patient's file because the latex agglutinates dry up, and i) is cost-effective compared to other sérodiagnostic methods.
- Thomas Oettinger, M.Sc.
Mycobacteria Department
Sector for Biotechnology
Statens Seruminstitut Copenhagen, Denmark and Scanpharm Ltd.
Department of Biotechnology
Ballerup, Denmark
- Ase B. Andersen, M.D.
Mycobacteria Department
Sector for Biotechnology
Statens Seruminstitut
Copenhagen, Denmark
- Ayenew Nurlign, B.Sc.
Armauer Hansen Research Institute
Addis Ababa, Ethiopia
- Peter Skinhoj, M.D.
Professor
Copenhagen University Hospital
Department of Infectious Diseases
Copenhagen, Denmark
- Sten Verland, Ph.D.
Scanpharm Ltd.
Department of Biotechnology
Ballerup, Denmark
Acknowledgment. This work was supported by a grant from the Academy for Technical Science, EF 384, to TO. The Armauer Hansen Research Institute (AHRI) is supported by the Norwegian and Swedish agencies for International Development (NORAD and SIDA) respectively, and by the Norwegian National Committee for Development of Research and Education (NUFU). We thank R. Gigg, National Institute for Medical Research, Mill Hill, London, U.K. for the chemically synthesized antigen ND-O-BSA and I. C. Bygbjerg, Copenhagen University Hospital, Department of Infectious Diseases, Copenhagen, Denmark for the Danish leprosy sera. We also thank Hanne Krygcr and Turid Christcnscn for technical assistance.
REFERENCES
1. BRYCESON, A. D. M. and PFALTZGRAFF, R. E. Leprosy. 3rd edn. Edinburgh: Churchill Livingstone, 1990, pp. 57-75.
2. BURGESS, P. J., FINE, P. E. M., PONNINGHAUS, J. M. and DRAPER, C. Serological tests in leprosy. The sensitivity, specificity and predictive value of ELISA test based on phenolic glycolipid antigens, and the implications for their use in epidemiological studies. Epidemiol. Infect. 101(1988)159-171.
3. CHANTEAU, S., CARTEL, J. L., CELERIER, P., PLI-CHART, R., DEFORGES, S. and Roux, J. PGL-I antigen and antibody detection in leprosy patients: evaluation under chemotherapy. Int. J. Lepr. 57(1989)735-743.
4. CHANTEAU, S., CARTEL, J-L., BOUTIN, J. P. and Roux, J. Evaluation of gelatin particle agglutination assay for the detection of anti-PGL-I antibodies; comparison with ELISA method and applicability on large scale study using blood collected on filter paper. Lepr. Rev. 62(1991)255-261.
5. CHO, S.-N., FUJIWARA, T., HUNTER, S. W., REA, T. H., GELBER, R. H., ASPINALL, G. O. and BREN- NAN, P. J. Use of an artificial antigen containing the 3, 6-di- O -methyl- β -D-glucopyranosyl epitope for the serodiagnosis of leprosy. J. Infect. Dis. 150(1984)311-322.
6. DYACHINA, M. N., LUKIN. Y. V., ZUBOV, V. P. and BOVIN, N. V. Microtiter particle agglutination test for diagnosis of leprosy. Int. J. Lepr. 60(1992)575-579.
7. FINE, P. E. M., PONNINGHAUS, J. M., BURGESS, P., CLARKSON, J. A. and DRAPER, C. C. Seroepidcmiological studies of leprosy in northern Malawi based on an enzyme-linked immunosorbent assay using synthetic glycoconjugate antigen. Int. J. Lepr. 56(1988)243-254.
8. GANJU, L., BATRA, H. V., TALWAR, G. P. and MUKHERJEE, R. A rapid agglutination test for detection of antibodies in tuberculosis and Hansen's disease. J. Immunoassay 12(1991)579-595.
9. IZUMI, S., FUJIWARA, T., IKEDA, M., NISHIMURA, Y., SUGIYAMA, K. and KAWATSU, K. Novel gelatin particle agglutination test for serodiagnosis of leprosy in the field. J. Clin. Microbiol. 3(1990)525-529.
10. JOHANSSON, S. G. O., MELLBIN, T. and VAHLQUIST, B. Immunoglobulin levels in Ethiopian preschool children with special references to high concentrations of immunoglobulin E (IgND). Lancet 1(1968)1118-1121.
11. LEFFORD, M. J., HUNEGNAW, M. and SIWIK, E. The value of IgM antibodies to PGL-I in diagnosis of leprosy. Int. J. Lepr. 59(1991)432-440.
12. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-275.
13. SPEARMAN, C. The proof and measurement of association between two things. Am. J. Psychol. 15(1904)72-101.
14. Wu, Q., YE, G., YIN, Y., LI, X., Liu, Q. and WEI, W. Rapid serodiagnosis for leprosy-a preliminary study on latex agglutination test. Int. J. Lepr. 58(1990)328-333.
15. YOUNG, D. B. and BUCHANAN, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
Reprint requests to: Thomas Oettinger, Mycobacteria Department, Sector for Biotechnology, Statens Seruminstitut, Artillerivej 5, DK-2300 Copenhagen S, Denmark. (Phone 45-32-68-37-71; fax = 45-32-68-38-71).
Present address for Dr. Verland: Mollegaard Breeding Centre Ltd, LI. Skensved, Denmark.