- Volume 61 , Number 4
- Page: 542–9
Case-control study of BCG vaccination as a risk factor for leprosy and tuberculosis in Western Kenya
ABSTRACT
A case-control study was carried out in western Kenya to measure the protection imparted by BCG against leprosy and tuberculosis. The study involved 69 newly diagnosed leprosy cases, 238 age-, sex- and neighborhood-matched controls, and 144 newly diagnosed, sputum-smear-positive tuberculosis cases along with 432 age-, sexand neighborhood-matched controls. Information on BCG vaccination history was inferred f rom scars. Using matched analysis, the protection imparted by BCG against leprosy was estimated to be 81% [95% confidence interval (CI) = 67-90] with no apparent difference in protection against paucibacillary [vaccine efficacy (VE) = 83%, 95% CI = 58-92] and multibacillary leprosy (VE = 76%; 95% CI = 30-91). The effectiveness against tuberculosis was appreciably lower (VE = 22%) and was not statistically significant (95% CI = -20-51).RÉSUMÉ
Une étude cas-témoins a été réalisée dans l'Ouest du Kenya dans le but de mesurer la protection apportée par le BCG vis-à-vis de la lèpre et de la tuberculose. L'étude comprenait 69 cas de lèpre nouvellement diagnostiqués, 238 témoins appariés pour l'âge, le sexe et le voisinage, ainsi que 144 cas de tuberculose nouvellement diagnostiqués, positifs à l'examen direct et 432 témoins appariés pour l'âge, le sexe et le voisinage. Les antécédents de vaccination par le BCG ont été déduits de la présence ou non de cicatrices. Sur base de tests paires, on a estimé la protection apportée par le BCG vis-à-vis de la lèpre à 81 % [intervalle de confiance à 95 % (IC): 67-90], sans différence apparente dans la protection vis-à-vis de la lèpre paucibacillaire [efficacité vaccinale (EV) = 83%; IC à 95% = 58-92] et la lèpre multibacillaire (EV = 76%; IC à 95% = 30-91]. L'efficacité vis-à-vis de la tuberculose était notablement plus basse (EV = 22%) et non statistiquement significative (IC à 95% = -20-51).RESUMEN
Se realizo un estúdio en Kenia occidental para medir la protección conferida por el BCG contra la lepra y la tuberculosis. El estúdio invólucro 69 casos de lepra recien diagnosticados y 238 controles sanos aparcados en cuanto a edad, sexo y residência, además de 144 pacientes con tuberculosis de reciente diagnóstico (con baciloscopías positivas) y 432 controles sanos aparcados también por edad, sexo y residência. La información sobre la vacunación previa con BCG se infirió de la presencia de cicatrices. Usando análisis aparcados se calculo que la protección conferida por el BCG contra la lepra fue dei 81% [intervalo de confianza al 95% (Cl) = 67-90], sin diferencias aparentes en la protección contra la lepra paucibacilar [eficiência de la vacuna (VE) = 83%; Cl al 95% = 58-92] y la lepra multibacilar (VE = 76%; Cl al 95% = 30-91). La efectividad contra la tuberculosis fue apreciablemente menor (VE = 22%) y no fue estadisticamente significativa (Cl al 95% = -20-51).The effect of the bacillus Calmette-Guerin (BCG) vaccine on mycobacterial diseases is still controversial despite the fact that it is one of the most widely used vaccines (4). Major field trials in Africa and elsewhere have failed to produce consistent results on the protection by BCG against leprosy and tuberculosis (14). A controlled trial undertaken in Uganda indicated 80% protection by BCG against tuberculoid leprosy (20). In Malawi (14), BCG has been found to impart 49% protection against paucibacillary leprosy and 84% protection against multibacillary leprosy. A household contact study in Togo (22) and a case-control study undertaken in Cameroon (2) both detected approximately 60% protection against tuberculosis. The study undertaken in Malawi failed to detect any protection of BCG against tuberculosis (14). Studies from other parts of the world have produced varying results, ranging from 20% to 60% against leprosy and 0 to 80% against tuberculosis (4,10,17). Only in Sout h India and (23) end Malawi (14) has the effectiveness of BCG been studied simultaneously against tuberculosis and leprosy in the same population. In both populations higher protection was found against leprosy than against tuberculosis.
Despite all of the controversies and the difficulties of demonstrating the impact of BCG at a population level, BCG is still given routinely to millions of children in developing countries, with the belief that it is preventing large numbers of cases of tuberculosis (or leprosy). It is important to assess the actual effect of this program on disease incidence.
In this paper we report the findings of a case-control study designed to measure the protective efficacy of BCG against tuberculosis and leprosy in Western Kenya.
MATERIALS AND METHODS
The study was undertaken in four districts of western Kenya known to be endemic for both leprosy and tuberculosis: Busia, Siaya, Kisumu and South Nyanza (9,12). The estimated population in the study area in 1989 was 3.3 million of whom 54% were younger than 15 years of age and 51% were females. Leprosy and tuberculosis control is coordinated in the study area by the National Leprosy and Tuberculosis Control Program (NLTP), an integrated control program run by the Kenya Ministry of Health. The programs are based on passive case finding and treatment. BCG was introduced into the study area in 1962 with mass vaccination campaigns concentrating on schools and market places. The official target population was children under 16 years of age (21,24). After the initial phase, which lasted almost 10 years, the BCG program was handed over to maternal and child health (MCH) welfare programs in 1972, and newborns and children less than 5 years old continued to receive BCG from MCH. In 1984, BCG was taken over by the Kenya Expanded Program on Immunization (KEPI), which now administers BCG vaccine to newborns and to children less than 5 years old (8).
As far as is known, most of the BCG used in the study area was Glaxo (freeze-dried). The recommended dosages were 0.05 ml for children less than 1 year old and 0.1 ml for children older than 1 year (0.1 ml = approximately 1.6 x 106 viable particles). The vaccine was injected intradermally into the lateral aspect of the left upper forearm, just lateral to the antecubital fossa.
For the purposes of this study, a leprosy case was defined as "a newly diagnosed, previously untreated, paucibacillary or multibacillary case with bacteriological and/or histopathological evidence of leprosy, aged between 0-65 years, resident in Busia, Siaya, Kisumu or South Nyanza districts, and diagnosed/registered for treatment after 1 April 1989." Neural leprosy cases were excluded from the study.
A tuberculosis case was defined as "a person newly diagnosed clinically and/or bacteriologically as having pulmonary tuberculosis within the health facilities and residents of Busia, Siaya, Kisumu and South Nyanza districts after 1 July 1989, and aged between 15-44 years."
Leprosy cases self-reported to the health units (dispensaries, health centers or district hospitals). Potential cases were recruited into the study at the time of registration or at first contact with the national control program. All of the identified cases were examined by the principal investigator (PAO) or one of the field officers (medical assistants specialized in tuberculosis and leprosy). The cases were then classified clinically according to the Ridley-Jopling scale, i.e., polar tuberculoid (TT), borderline tuberculoid (BT), borderline borderline (BB), borderline lepromatous (BL), and polar lepromatous (LL). These cases were also subcategorized by diagnostic certainty according to predetermined guidelines. Cases were subclassified clinically as "certainly" leprosy, "most likely" leprosy or "possibly" leprosy. Each case had a skin biopsy taken and sent to one of us (SBL) for histopathological examination. The interpretation of the skin biopsy was done according to guidelines developed in Malawi (5).
Tuberculosis cases were self-reporting patients who had initially been identified by the control program and whose sputum was positive by direct microscopy. Another sputum sample was collected and sent to the reference laboratory in Nairobi for repeat direct examination and culture. At the reference laboratory, direct microscopy was done using the auramine-phenol technique and reported as "negative," "scantly positive," "moderately positive" or "heavily positive." The same sputa were cultured and the results reported within 6-8 weeks as "negative," "contaminated," "scanty," "moderately positive" or "heavily positive." The identification of culture isolates as Mycobacterium tuberculosis also was done at the reference laboratory.
Controls were selected by identifying and examining inhabitants of households near that of the case. The principal investigator (PAO) or field officers canvassed the neighborhood of cases following predetermined rules until appropriate age- and sex-matched controls were identified. Once a potential control was identified, the purpose of the study was explained to him or her. If s/he agreed to participate, s/he was then examined to rule out tuberculosis or leprosy. Any potential control who appeared to have leprosy was excluded as a control for the leprosy study (one control was excluded on this basis). Any control with a history of chronic cough was excluded as a control for tuberculosis.
All cases and controls were examined for the presence of a BCG scar and questioned about socioeconomic characteristics, such as type of house, household items, occupation, land ownership, distance from the nearest health unit, crops grown by the family, and level of education of the head of the household and the respondent.
Exposure to BCG was ascertained initially by the principal investigator or one of the field officers who were blind neither to the study hypothesis nor to the case or control status. BCG scars were later read independently by field assistants (paramedical health workers) trained in the reading of BCG scars. Evidence of a BCG scar was recorded as present, absent, doubtful or unknown. Field assistants were kept blind to the study hypothesis throughout the duration of the study. An effort was also made to keep them blind to the case or control status of the study subjects by involving them in the collection of information on smallpox scars, collection and examination of urine on the spot using chemical strips (URISTIX, Emmes, U.K.), and collection and parasitological examination of stools. The largest transverse diameter of any scar was measured and recorded in millimeters.
Data analysis was conducted using Statistical Analysis System (SAS) computer software (SAS Institute, Inc., Cary, North Carolina, U.S.A., 1985), and EGRET (SER-EC, Seattle, Washington, U.S.A.). Conditional likelihood estimates (CMLE) and their 95% confidence intervals were transformed into disease exposure odds ratios and confidence limits.
RESULTS
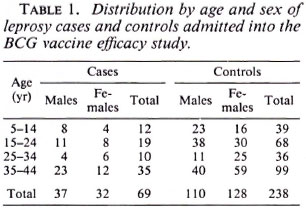
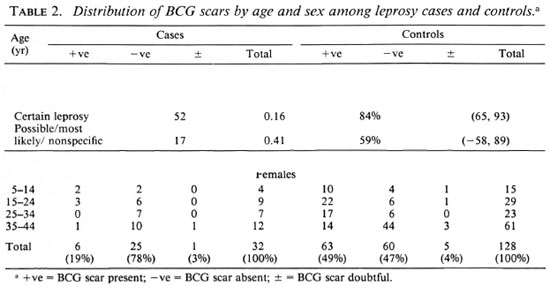
Of 182 potential leprosy cases ascertained by the routine leprosy control program, 132 were enrolled into the study. Of these cases, 131 were biopsied; 42 (32%) had skin smears taken (by the control program). Of the 132 total cases, three who satisfied both clinical and histopathological criteria were excluded from the study because suitable controls could not be identified for them. This left 129 leprosy cases for whom 384 controls were identified. The major analysis was restricted to those who were 44 years of age or younger since older individuals were born too early to have been included in the BCG programs. The distribution of the 69 leprosy cases and 238 controls included in these analyses is shown in Table 1; the distribution of BCG scars among the cases and controls is shown in Table 2.
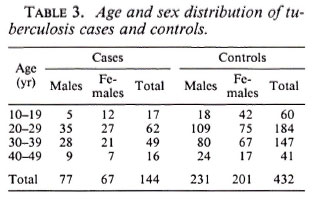
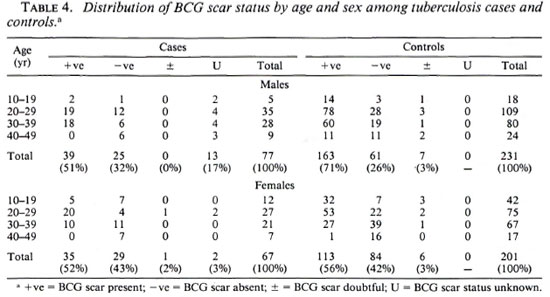
Among the 144 eligible tuberculosis cases, 13 (9%) did not have sputum sent to the reference laboratory for logistic reasons, and 11 (7.6%) were both microscopy and culture negative at the reference laboratory. A certainty group subclassification was established to define "certain" tuberculosis (those who were repeat smear and/or culture positive) and "possible" tuberculosis (those who were negative to repeat smear or culture, and those who did not have sputum sent to the central laboratory). A further 15 cases had to be excluded since they could not be found by field assistants. Following these criteria, there were 119 "certain" and 25 "possible" tuberculosis cases included in the analysis (Table 3). The distribution of BCG scar status among tuberculosis cases and controls is shown in Table 4.
In order to take matching of controls and additional potential confounding into account, multiple conditional logistic regression was performed. The estimated vaccine efficacy of BCG against leprosy is shown in Table 5; against tuberculosis in Table 6. The overall protective efficacy of BCG against leprosy was found to be 81% and highly statistically significant (95% CI = 67, 80) with some evidence (not statistically significant) for higher efficacy among older individuals and among females. Among tuberculosis cases, the point estimate overall vaccine efficacy was found to be 22%, but this was not statistically significantly greater than zero (95% CI = -20 , 51). This estimated vaccine efficacy appeared to have no age or sex trend.
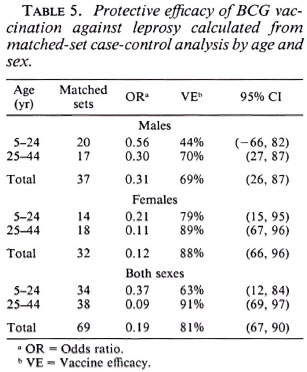
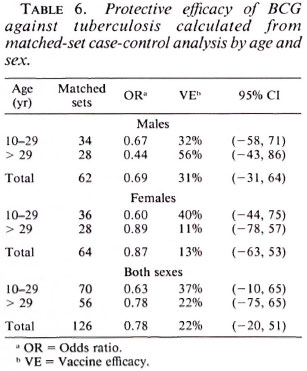
Table 7 shows BCG vaccine efficacy against leprosy separated by histopathological classification and diagnostic certainty. The vaccine appeared to give similar protection against all clinical forms of the disease. When cases were classified into two broad categories of paucibacillary (TT + BT), and multibacillary leprosy (BB + BL + LL), the protective effects were 83% (95% CI = 85, 92) and 76% (95% CI = 30, 91), respectively, suggesting that BCG was equally protective against paucibacillary and multibacillary leprosy. Higher efficacy (84%) was noted against "certain" than against "most likely/possible/unknown" disease of western Kenya. This is consistent with (59%).
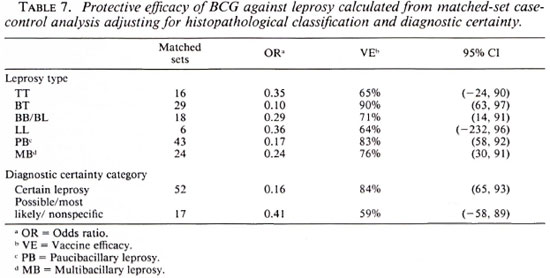
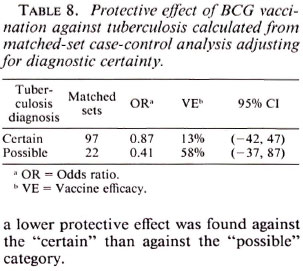
Table 8 shows vaccine efficacy estimates against tuberculosis when broken down by diagnostic certainty. Surprisingly, although the estimates were not statistically different, a lower protective effect was found against the "certain" than against the "possible" category.
DISCUSSION
The results presented here are based on a case-control design with self-presenting leprosy and tuberculosis cases and age-, sex-and neighborhood-matched controls. There was no active case detection in the study area, thus cases in this investigation were representative of those seen within the control program.
This investigation provides evidence thatBCG gives stronger protection against leprosy than against tuberculosis in this area of western Kenya. This is consistent with findings in other areas where BCG efficacy against tuberculosis and leprosy have been evaluated concurrently. In South India (23) the protective efficacy was found to be 0% and 23% for tuberculosis and leprosy, respectively.
In Malawi (7,14) the protection was found to be 0% and above 50% for tuberculosis and leprosy, respectively. Estimates of BCG protection obtained in the present study were 22% for tuberculosis and 81% for leprosy.
This difference is unlikely to be due to methodological errors for several reasons: a) BCG scar status was assessed by field assistants who were purposely kept blind of the case or control status, and the study hypothesis. This should have minimized any potential information bias, and b) we took precautions in ensuring the accuracy of diagnoses. Since it is widely recognized that the diagnosis of leprosy is difficult, strict criteria were developed for the diagnosis of leprosy in the field, and also histopathologically. Cases of childhood tuberculosis were not included in this study.
Another possible source of error is the misclassification of BCG scars (6,8). Inasmuch as BCG may not leave a scar or a scar may be missed on examination, random or nonrandom misclassification could be introduced into the study. In Kenya (8) it was found in one KEPI survey that 5% of the children who had received vaccination at birth did not have BCG scars 20 months later. An independent study to estimate the sensitivity and specificity for BCG scar reading in the context of the present investigation detected that 14% of the children who had documented evidence of prior BCG vaccination did not have BCG scars on examination. This implies a nondifferential misclassification, which would have lowered the estimated protective effect. The sensitivity and specificity of BCG scar reading in this study were estimated to be 86.4% and 97.4%, respectively. Fine, et al. (6) have noted from their work in Malawi that a sensitivity of 90% and specificity of 90% would lead to about 11% underestimation of a vaccine's efficacy. Since vaccine efficacy depends mainly upon the specificity of BCG scar reading (15), the "true" efficacy may have been underestimated in this study by about 5%.
The protective efficacy of BCG versus leprosy in the study population was found to be high. The protective efficacy was found to be higher against borderline leprosy than against polar forms of leprosy, but did not differ between multibacillary and paucibacillary disease. Protection against the multibacillary form of leprosy also has been found in several studies (10,11,14,23) , and has significant public health implications because multibacillary disease is thought to be a major source for the spread of M leprae in the community.
This study detected a nonsignificant 22% protective effect of BCG against tuberculosis. While the finding was consistent with estimates obtained from some parts of the world (13,16,18,19), it differs from studies in West Africa (2,22) which detected 60% protection.
Among the possible explanations for the low protective efficacy of BCG against tuberculosis obtained in this study are: a) overmatching on some socioeconomic variables which were associated strongly with BCG exposure, which could have existed and were not measured; b) the existence of atypical mycobacteria in the study area which would have lowered the observable protective efficacy (7) and/or c) a preponderance of exogenous reinfection tuberculosis against which BCG may be able to play only a minor role.
We emphasize that the vaccine effects identified in this study reflect effects against passively ascertained disease, which tends to be more advanced than disease identified in active case-detection surveys. Considering our results by classification and diagnostic certainty (Table 7), there is evidence for strong protection against BT disease, as noted in New Guinea (1), but little evidence for a general shift of the disease from multibacillary to paucibacillary and indeterminate forms as observed in South India (11).
There is some evidence from this study, and from Malawi (14) , of an inverse relationship between BCG protection against tuberculosis and against leprosy. Therefore, it would be prudent to conduct further casecontrol studies in situations similar to ours in order to determine the regional variability and other determinants of BCG's effects.
Acknowledgment. This study was supported by a grant from the Special Programme for Research and Training in Tropical Diseases (TDR) of the UNDP/ World Bank/World Health Organization. We are grateful to GPA/WHO for partially funding the field work. The authors are grateful to Drs. J. Omwega, formerly Director, Respiratory Diseases Research Center, and B. Swai for their assistance. We are also thankful to Dr. Jonathan Sterne for help in data analysis. Last but not least, the authors are grateful to the Director, Kenya Medical Research Institute (KEMRI), for his permission to publish this article.
REFERENCES
1. BAGSHAWE, A., SCOTT, G. C, RUSSELL, D. A., WIGLEY, S. C, MERIANOS, A. and BERRY, G. BCG vaccination in leprosy: final results of the trial in Karimui, Papua New Guinea. Bull. WHO 67(1989)389-399.
2. BLIN, P., DELOLME, H. G., HEYRAUD, J. D., CHAR-PAK, Y. and SENTILHES, L. Evaluation of protective effect of BCG vaccination by case control study in Yaounde, Cameroon. Tubercle 67(1986)283-288.
3. CENTRAL BUREAU OF STATISTICS Population Projections for Kenya 1983-2000. Kenya National Census 1979 Ministry of Planning and National Development, 1983.
4. FINE, P. E. M. BCG vaccination against tuberculosis and leprosy. Br. Med. Bull. 44(1988)691-703.
5. FINE, P. E. M., JOB, K., MCDOUGALL, A. C, MEYERS, W. M. and PONNIGHAUS, J. M. Comparability among histopathologists in the diagnosis and classification of lesions suspected of leprosy in Malawi. Int. J. Lepr. 54(1986)614-625.
6. FINE, P. E. M., PONNIGHAUS, J. M. and MAINE, N. The distribution and implication of BCG scars in Northern Malawi. Bull. WHO 67(1989)35-41.
7. FINE, P. E. M., PONNIGHAUS, J. M., MAINE, N., CLARKSON, J. A. and BLISS, L. Protective efficacy of BCG against leprosy in northern Malawi. Lancet 2(1986)499-502.
8. KENYA EXPANDED PROGRAMME ON IMMUNIZATION MANAGEMENT UNIT. National Immunization Coverage Survey (1987). Ministry of Health, Kenya.
9. LEIKER, D. L., OTSYULA, Y. and ZIEDSES DES PLANTES, M. Leprosy and tuberculosis in Kenya. Lepr. Rev. 39(1968)79-83.
10. LWIN, K., SUNDARESAN, T., GYI, M. M., BECHELLI, L. M., TAMONDONG, C, GARBAJOSA, P. G., SANSARHICQ, H. AND NOORDEEN, S. K. BCG vaccination of children against leprosy; 14 year finding of the Burma trial. Bull. WHO 63(1985)1069-1078.
11. Muliyil, J., Nelson, K. E. and DIAMOND, E. L. The effect of BCG on the risk of leprosy in an endemic area: a case control study. Int. J. Lepr. 59(1991)229-236.
12. NATIONAL LEPROSY AND TUBERCULOSIS CONTROL PROGRAMME (NLTR). Western and Nyanza Provinces, Annual Report (1986). Ministry of Health, Kenya.
13. PATEL, A., SCHOFIELD, F., SISKIND, V., ABRAHAMS, E. and PARKER, J. Case control evaluation ofschool age BCG vaccination programme in subtropical Australia. Bull. WHO 69(1991)425-433.
14. PÖNNIGHAUS, J. M., FINE, P. E. M., STERNE, J. A. C, WILSON, R. S., MSOSA E., GRUER, P. J. K., JENKINS, P. A., LUCAS, S. B., LIOMBA, G. and BLISS, L. Efficacy of BCG against leprosy and tuberculosis in northern Malawi. Lancet 339(1992) 636-639.
15. RADHAKRISHNA, S., NAIR, N. G. K. and JAYABAL, P. Implications of misdiagnosis in field trials of vaccines. Indian J. Med. Res. 80(1984)711-720.
16. Shapiro, C, Cook, N., Evans, D., Willct, W., Fajarda, L, Koch-Weser, D., Bergonzoli, G., Bolanos, O., Guerrero, R. and HENNEKENS, C. A case control study of BCG and childhood tuberculosis in Cali, Colombia. Int. J. Epidemiol. 14(1985)441-446.
17. SMITH, P. G. Case control studies of the efficacy of BCG against tuberculosis: Tuberculosis and Respiratory Diseases. Papers presented at the XX Vlth World Conference of International Union Against Tuberculosis, Singapore, 4-7 Nov 1986.) Professional Postgraduate Services, Japan 1987, pp. 73-79.
18. SMITH, P.G. Case control studies of the efficacy of BCG vaccine in children. Bull. Int. Union Tubercul. Lung Dis. 62(1987)70-76.
19. SMITH, P. G. Evaluating interventions against tropical diseases. Int. J. Epidemiol. 16(1987)159-166.
20. STANLEY, S. J., HOWLAND, C, STONE, M. M. and SUTHERLAND, I. BCG vaccinations against leprosy in Uganda: final results. J. Hyg. (Cambridge) 87(1981)233-248.
21. SWAI, O. B., ALUOCH, J. A., KAMUNVI, F., AG-WADA, R., KWAMANGA, D. and KENYA, P. R. National BCG scar survey in the estimation of BCG vaccination in Kenya. East Afr. Med. J. 62(1985)842-851.
22. TIDJANI, O., AMEDONE, A. and TEN DAM, H. G. The protective effect of BCG vaccination of the newborn against childhood tuberculosis in an African community. Tubercle 67(1986)269-281.
23. TRIPATHY, S. P. The case for BCG. Ann. Natl. Acad. Med. Sci. (India) 19(1983)12-21.
24. TUBERCULOSIS AND RESPIRATORY DISEASES RESEARCH CENTRE, KENYA MEDICAL RESEARCH INSTITUTE. Report of Kenya National Tuberculosis Survey 1979-1981, Phase 1; Ministry of Health, Kenya, 1984.
1. M.B.Ch.B., Ph.D.; Kenya Medical Research Institute, Alupe Leprosy and Skin Diseases Research Center, P.O. Box 3, Busia, Kenya.
2. Dip.Clin.Med.; Kenya Medical Research Institute, Alupe Leprosy and Skin Diseases Research Center, P.O. Box 3, Busia, Kenya.
3. Dip.Clin.Med.; Kenya Medical Research Institute, Alupe Leprosy and Skin Diseases Research Center, P.O. Box 3, Busia, Kenya.
4. Cert. Community Nurse, Kenya Medical Research Institute, Alupe Leprosy and Skin Diseases Research Center, P.O. Box 3, Busia, Kenya.
5. V.M.D., Ph.D., Department of Epidemiology and Population Sciences, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, U.K.
6. F.R.C.P., F.R.C. Path., University College and Middlesex School of Medicine, Gower Street, London WC1E 6JJ, U.K.
Received for publication on 1 October 1992;
Accepted for publication in revised form on 24 August 1993.