- Volume 61 , Number 4
- Page: 533–41
Low predictive value of PGL-I serology for the early diagnosis of leprosy in family contacts: results of a 10-year prospective field study in French Polynesia
ABSTRACT
In 1983, a cohort study to follow up the family contacts of leprosy cases was implemented in French Polynesia to assess the usefulness and applicability of phenolic glycolipid-I (PGL-I) serology in a leprosy control program. A total of 1201 contacts (666 females, 535 males) have been included in the study. The IgM anti-PGL-I seroprevalence determined on the initial sera was 17%. It was significantly higher among females than males (20% vs 15%, p = 0.02). From 1983 to 1992, 4 out of 204 (2%) anti-PGL-I seropositive contacts developed the disease (1 indeterminate, 1 BT, 1 BL, 1 LL) compared with 10 out of 997 (1%) seronegative contacts (4 indeterminate, 3 BT, 1 BB, 2 TT). Of these 10 patients, only 3 (2 indeterminate, 1 BT) converted to seropositivity when leprosy was diagnosed. The risk of developing leprosy was not significantly higher among seropositive than among seronegative groups (2% vs 1%, p = 0.2). A PGL-I circulating antigen test performed on 216 selected sera at entry into the trial showed a higher antigen prevalence when the antibody level was higher. PGL-I antigen was detectable in 5 of 12 patients tested prior to diagnosis (1 LL, 1 BL, 3 indeterminate). The median time to externalize the disease was not significantly different among antibody-positive and -negative contacts (17 vs 25 months, p = 0.3). The relative risk of developing leprosy for contact individuals was 30.8 times that of noncontacts, and 15% of the total new cases detected between 1983 and 1992 emerged f rom the study population. In conclusion, this 10-year prospective study clearly shows that IgM anti-PGL-I serology is not effective for the early diagnosis of leprosy among a high-risk population. Therefore, in most of the endemic countries this test cannot be recommended for a leprosy control program.RÉSUMÉ
En 1983, une étude de cohortes de surveillance des contacts familiaux de malades de la lèpre a été entreprise en Polynésie française pour estimer l'utilité et l'applicabilité d'un test sérologique basé sur Je glycolipide phénolique (GLP-I) dans un programme de lutte contre la lèpre. Un total de 1201 contacts (666 femmes et 535 hommes) a été incorporé dans l'étude. Le taux de séroprévalence des anticorps IgM anti-GLP-I déterminé sur les serum de départ était de 17%. Il était significativement plus élevé chez les femmes que chez les hommes (20% contre 15%, p = 0.02). De 1983 à 1992, 4 des 204 (2%) contacts séropositifs vis-à-vis du GLP-I ont développé la maladie (1 indéterminé, 1 BT, 1 BL et 1 LL), comparés à 10 parmi 997 (1%) contacts séronégatifs (4 indéterminés, 3 BT, 1 BB, 2 TT). Parmi ces 10 patients, seulement 3 (2 indéterminés, 1 BT) ont viré vers la séropositivité quand la lèpre a été diagnostiquée. Le risque de développer la lèpre n'était pas significativement plus élevé parmi les séropositifs que parmi les séronégatifs (2% contre 1%, p = 0.2). Un test pour rechercher l'antigène circulant GLP-I réalisé sur 216 serums sélectionnés à l'entrée dans l'étude a montré une prevalence plus élevée de l'antigène quand le taux d'anticorps était plus élevé. L'antigène GLP-I était détectable chez 5 des 12 patients testés avant le diagnostic (ILL, 1BL, 3 indéterminés). Le temps médian d'extériorisation de la maladie n'était pas significativement différent entre les contacts positifs pour les anticorps et ceux négatifs (17 contre 25 mois, p = 0.3). Le risque relatif de développer la lèpre pour des contacts individuels était 30.8 fois plus élevé que pour des noncontacts, et 15 % du total des nouveaux cas détectés entre 1983 et 1992 provenaient de la population d'étude. En conclusion, cette étude prospective de 10 ans montre clairement que la sérologie basée sur les anticorps IgM vis-à-vis du GLP-I n'est pas efficace pour le diagnostic précoce de la lèpre dans une population à haut risque. Par conséquent, dans la plupart des pays endémiques, ce test ne peut être recommandé dans le cadre d'un programc de lutte contre la lèpre.RESUMEN
Con el fin de establecer la utilidad y la aplicabilidad de la serologia del glicolípido fenólico (PGL-I) en un programa de control contra la lepra, en 1983 se implemento en la Polinesia Francesa un estudio para el seguimiento de los contactos familiares de pacientes con lepra. En el estudio se incluyeron un total de 1201 contactos (666 mujeres, 535 hombres). La seroprevalencia de anticuerpos IgM anti-PGL-I en los sueros iniciales fue del 17%, y fue significativamente mayor entre las mujeres que entre los hombres (20% vs 15%, p = 0.02). De 1983 a 1992, 4 de 204 (2%) contactos anti-PGL-I positivos desarrollaron la enfermedad (1 indeterminado, 1 BL, 1 LL) mientras que esto ocurrió en 10 de 997 (1%) contactos seronegativos (4 indeterminados, 3 BT, 1 BB, 2 TT). De estos 10 pacientes sólo 3 (2 indeterminados, 1 BT) adquirieron la seropositividad después de que se diagnosticó la enfermedad. El riesgo de desarrollar la lepra no fue significativamente mayor entre los grupos seropositi vos que entre los seronegativos (2% vs 1%, p = 0.2). Una prueba para medir PGL-I circulante en 216 sueros seleccionados al momento de iniciar el estudio mostró una mayor prevalencia del antígeno cuando el nivel de anticuerpo fue más alto. El PGL-I fue détectable en 5 de 12 pacientes probados antes de establecer el diagnóstico (1 LL, 1 BL, 3 indeterminados). El tiempo promedio para manifestar la enfermedad no fue significativamente diferente entre los contactos anticuerpo-positivos y aquellos anticuerpo-negativos ( 17 vs 25 meses, p = 0.3). El riesgo relativo de desarrollar la enfermedad en los individuos contacto fue 30.8 veces mayor que el de los no contactos. Quince porciento del total de casos nuevos detactados entre 1983 y 1992 emergieron de la población estudiada. En conclusión, este estudio prospectivo de 10 años muestra claramente que la serología para IgM anti-PGL-I no es efectiva para el diagnóstico temprano de la lepra en una población de alto riesgo. Por lo tanto, en la mayoría de los países endémicos esta prueba no puede ser recomendada para aplicarse en los programas de control de la lepra.There is enough evidence to indicate that subclinical infections in leprosy are far more prevalent than manifest disease, and that a very large proportion of subclinical infections is aborted (1,12,14,17) . Preliminary serological studies in the early 1980s, based on the detection of antiphenolic glycolipid-I (anti-PGL-I), have generated considerable interest in the study of subclinical infection in leprosy (7,15,23). The application of this test toward the identification of early disease in a high-risk population has been implemented in many leprosy-endemic countries as reviewed by Fine (10). In 1983, a prospective cohort study of a population of family contacts was launched in French Polynesia, to assess the usefulness, the feasibility and the predictive value of the ELISA IgM anti-PGL-I for the diagnosis of early leprosy under field conditions. The preliminary results over a 2-year period in the past suggested the possible usefulness of this test for the prediction of multibacillary leprosy (6). In the present paper, we report the results obtained after 10 years of follow up.
MATERIALS AND METHODS
Population studied. French Polynesia is composed of 120 islands spread over an area of 4 million sq. km. The mean population for the period 1982-1992 was 188,020 inhabitants, of whom two thirds live in Tahiti, the main island. The leprosy control program is supervised by Malarde Institute. Multidrug therapy including daily administration of 10 mg/kg of rifampin in addition to dapsone and clofazimine in multibacillary (MB) leprosy, and in addition to dapsone in paucibacillary (PB) leprosy, has been implemented since 1982. The mean annual case detection rate during the period 1982-1992 was 6.3 per 100,000 (3).
All known family contacts of leprosy cases were eligible for the study. They underwent a physical examination for determination of leprosy, and blood samples were taken for serology.
Diagnosis of leprosy patients. The diagnosis of leprosy was based on the results of clinical examination, complemented by biological tests: an intradermal lepromin reaction, acid-fast bacilli (AFB) in the nasal mucosa and the skin (lesion and two earlobes), and a biopsy for histopathological examination (P. Ravisse and M. Huerre, Institut Pasteur, Paris, France). The patients were classified according to Ridley and Jopling's classification (19).
The physicians responsible for the diagnosis of leprosy were not aware of the serological results, and the laboratory technician did not receive clinical information throughout the study.
IgM anti-PGL-I detection. An indirect ELISA for IgM anti-PGL-I antibodies was conducted using a previously described procedure (5). The antigen used was the synthetic analog of PGL-I, the trisaccharide 3-phydroxy-phenyl-propionate (NTP) coupled to bovine serum albumin (Lot Fuji-XI-66860717; Institute for Natural Science, Nara University, Japan). A serum was considered positive at 1/250 dilution when the optical density (OD 492 nm) exceeded the threshold of 0.200, which was the mean OD obtained from 400 sera from healthy Polynesians plus two standard deviations (S.D.) (specificity 97.5%). The sensitivity was 95% for MB patients (107 sera tested) and 35% for PB patients (61 sera tested).
Circulating PGL-I antigen detection. The detailed methodology has been described previously (5). PGL antigen in serum was extracted from 0.5 ml of serum, purified by chromatography on a Florisil column and semiquantitated by Dot-ELISA on a nitrocellulose membrane. As a standard, a known amount of purified PGL-I antigen (kindly provided by Dr. P. J. Brennan) was serially diluted. A serum was considered positive for PGL-I when the concentration was above 12 ng/ml. The specificity determined on 85 sera from healthy Polynesian individuals was 100%. The sensitivity of the test was 100% in MB patients (65 sera tested) and 20% in PB patients (50 sera tested).
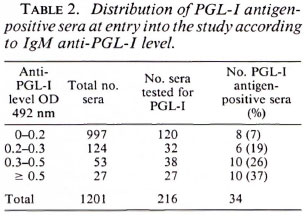
Because antigen detection is time consuming, this test was not performed on all of the sera. It was performed on all of the anti-PGL-I sera with which the OD was > 0.3, on one fourth of the positive sera with which the OD was between 0.2 and 0.3, and on a random selection of 120 out of 997 negative sera.
Statistical analysis. The percentages were compared using the chi-squared or Fischer test and the medians using the Mann-Whitney test. All p values lower than 0.05 were considered statistically significant.
RESULTS
Demography. From 1983 to 1992, 1201 family contacts of leprosy patients were included in a prospective PGL-I serological study: 666 were females and 535 were males, with a total of 8195 person-years. The distribution of family contacts included, according to the year of inclusion, is represented in Figure 1; most of the individuals (53%) were recruited in 1984. The age distribution of this population is shown in Figure 2 and is roughly similar to the age distribution of the total population.
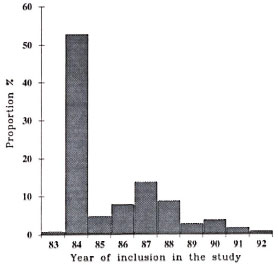
Fig. 1. Proportion of family contact individuals included in the study between 1983 and 1992 (total 1201 subjects) by year of inclusion.
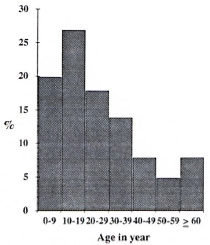
Fig. 2. Age distribution of 1201 studied family contacts.
Of the 1201 contacts, 433 (36%) were tested several times during the study (from 2 to 8 times).
Between 1984 and 1992, leprosy was detected in 14 out of the 1201 individuals, giving an incidence rate of 171 /100,000 person-years. As shown in Table 1, the relative risk of leprosy in contacts was 30.8 times that of noncontacts.

Antibody results at entry and evolution during the study. The distribution of IgM anti-PGL-I levels determined on the 1201 initial sera at entry into the study is shown in Figure 3. Using a cut-off of 0.200, the global positive seroprevalence of the contact population studied was 17%. It was significantly higher in females (20%) than in males (15%, p = 0.02).
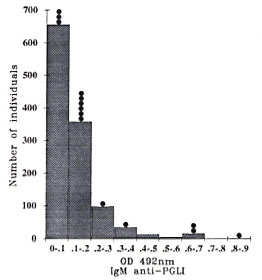
Fig. 3. Distribution of IgM anti-PGL-I levels in 1201 contacts at entry into the study;  = leprosy cases diagnosed during the study.
= leprosy cases diagnosed during the study.
Figure 4 shows the seroprevalence according to age group and sex. In both sexes, the seroprevalence increased rapidly up to the age group between 10 and 19 years old, at which point it decreased steadily down to the older age groups.
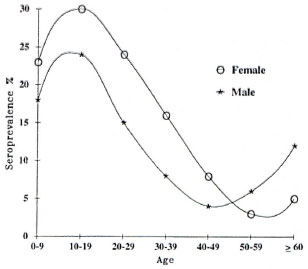
Fig. 4. IgM anti-PGL-I seroprevalence in familycontact population according to age group and sex;  = females; (*) = males.
= females; (*) = males.
Out of the 1201 individuals, excluding the 14 individuals who subsequently developed the disease, 433 contacts could be retested at least two times. The proportion of seropositive individuals among the retested individuals was significantly higher than among the 768 nonretested contacts (28% versus 11%, p < 0.005), mainly because more efforts to recontact them were focussed on the seropositive group. During the study, 28 out of the 121 antibody seropositive contacts (23%) spontaneously reverted to seronegativity; whereas 26 of the 312 seronegative subjects (8%) converted to seropositivity. The median age of individuals whose serological status changed was 17 years, not significantly different from the median age of individuals whose status did not change (18 years).
Antigen results and evolution during the study. The PGL-I antigen detection performed on the initial sera are shown in Table 2. The proportion of PGL-I positive sera tended to increase in sera containing higher anti-PGL-I levels. Excluding the subjects who externalized leprosy, the serum PGL-I level in the 31 positive individuals varied from 12 to 100 ng/ml. Among those individuals, 24 were followed regularly and are still leprosy free; 22 of them (91%) showed a spontaneous negativation, 2 remained positive at a stable level.
Development of clinical leprosy. Between 1984 and 1992, of the 14 patients who developed clinical leprosy, 4 came from the 204 initially antibody seropositive (one indeterminate [I], 1BT, 1BL, ILL) and 10 from the 997 initially seronegative individuals (41, IBB, 3BT, 2TT). The risk of developing the disease was not significantly higher among antibody-seropositive than -seronegative groups (2% versus 1%, p = 0.2). For each of these 14 patients, the variation of the anti-PGL-I antibody level on serial sera collected before the diagnosis of the disease is shown in Figure 5. Of the 10 seronegative individuals, seroconversion was observed for only 3 of them (21, 1BT).
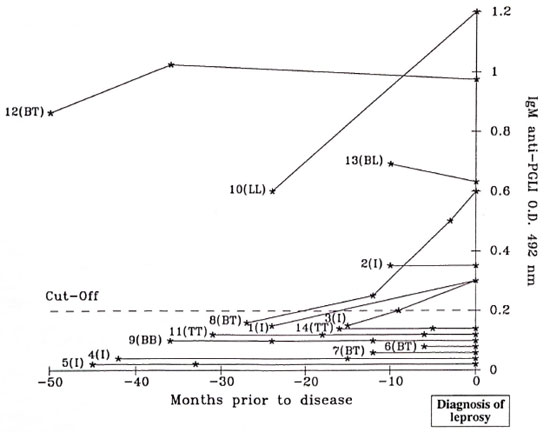
Fig. 5. Evolution of lgM anti-PGL-I in 14 contacts prior to diagnosis of leprosy; (*) = a serological determination.
The median time of participation was 100 months for seronegative and 93 months for seropositive groups. The median times to develop leprosy, although longer for seronegative than for seropositive individuals, were not significantly different (25 months for the seronegative versus 17 months for the seropositive patients).
PGL-I antigen detection could be determined for 12 of the 14 contacts who developed leprosy. For each patient, the antigen level evolution on serial sera prior to the diagnosis of leprosy is represented in Figure 6. At entry into the trial, three of these contacts were antigen positive and developed leprosy between 10 and 24 months (ILL, 1BL, II). During the study, two of these individuals seroconverted for PGL-I antigen and leprosy was diagnosed 3 months and 18 months later (21). At the time of the diagnosis, PGL-I antigen was thus positive for 5 patients (ILL, 1BL, 31).
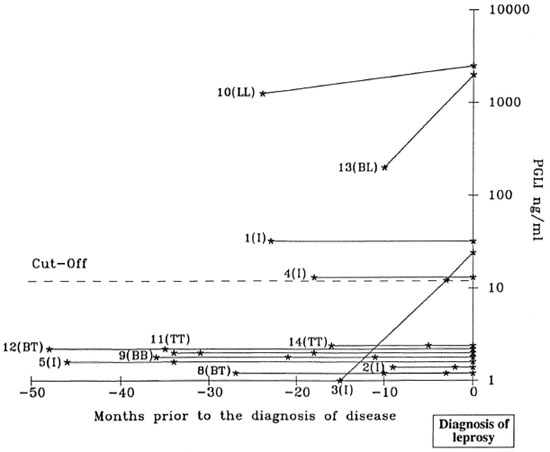
Fig. 6. Circulating PGL-I antigen evolution in 12 contacts prior to diagnosis ofleprosy; (*) = a serological determination.
DISCUSSION
Several serological studies on contact populations living in areas with different leprosy case-detection rates have been reported. Depending on the prevalence of the disease, the global anti-PGL-I seroprevalence among contacts was either higher or not than in the general population. In Papua New Guinea (2) and in Malawi (10), the seroprevalence was similar among household and nonhousehold individuals, probably because of the particular social organization in these countries and the high level of transmission of M. leprae in the whole community. In other studies, IgM anti-PGL-I global seroprevalence usually ranged from 5% to 30% and was higher than for the corresponding general population as is the case in the present study in French Polynesia. This seroprevalence was significantly higher in females (20%) than in males (15%), and the seropositivity was more prevalent in the age group 10-19 years old. Beyond this age group, there was a marked decline in seroprevalence with increasing age. This observation seems to be a general situation since it also has been reported in the total populations (4) as well as in the contact populations in many of the leprosy-endemic countries, such as Papua New Guinea (2), Malawi (11), Indonesia (20) and Venezuela (22). The reason why the specific IgM anti-PGL-I level was higher among females in all age groups remains unclear. Perhaps this difference may be attributed to the higher total IgM level in females than in males. (16).
During the course of the study in the population that could be retested several times, 23% of the antibody-positive contacts and 91% of the antigen-positive contacts became negative. The significance of these antibody and antigen reversions is not known, and may reflect an effective immune defense of the host resulting in the elimination of the bacilli or in a subclinical progression toward a paucibacillary form of leprosy. On the other hand, 77% of the antibody-positive individuals and 9% of the antigen-positive individuals remained positive for many years without clinical leprosy. The hypothesis of a very slow, progressive, subclinical infection or of a nonspecific background may be suggested. Over the total period of the survey, antibody seroconversion was observed in a total of 8% of the individuals who could be retested. Presuming that the conversion to seropositivity corresponds to infection, the rate of acquisition of infection would be much less than what was observed (9.5% annual rate) in Papua New Guinea (2). The difference could correspond to the large difference in the annual case detection rates between the two countries (6.3/100,000 versus 550/100,000). In both studies, the changes in anti-PGL-I status (conversion or reversion) occurred more frequently in subjects younger than 20 years of age than in older ones. This is compatible with the acquisition of M. leprae infection at a young age, and the subsequent decrease of the proportion of susceptible individuals in older age groups.
Of the 14 leprosy cases detected in the study group, 4 emerged from the antibodypositive group and 10 from the antibody negative group, among whom only 3 seroconvcrted before the diagnosis of the disease. Therefore, one half of the leprosy cases diagnosed during the course of the study remained antibody-negative. About the same proportion of seronegative leprosy patients (65%) has been reported from a largescale study in Venezuela (22). This observation is not surprising since most of these new patients were paucibacillary patients and the sensitivity of the test for the diagnosis of paucibacillary patients is low (20% to 50%). As far as we know, cohort studies on contact populations using a PGL-I antigen-detection assay are scarce. Before the diagnosis of leprosy, antigen tests were positive in 2 lepromatous (ILL and 1BL) and 1 indeterminate leprosy case. The same comment may be addressed to the antigendetection assay regarding its sensitivity for the diagnosis of paucibacillary patients.
The present study is one of the few in which it has been possible to assess under field conditions for a long period of time the predictive value of a PGL-I antibody assay for future risk of developing leprosy. In our previous preliminary report (6), and similar to other reports concerning short-term studies (8.22), a strong association was found between a high IgM anti-PGL-I level and the subsequent risk to developing the disease. Our present data, obtained after nearly 10 years of observation, finally concluded that there is a nonsignificant difference in the risk of developing leprosy whether the contacts were anti-PGL-I positive or negative. Such disappointing results have been reported previously by Groenen, et al. (13). The vast majority of seropositive subjects did not, at least in the time frame of our study, develop the disease. In other words, the predictive value of a positive anti-PGL-I result was very low.
However, it is important to note the high proportion (15%) of leprosy patients who emerged from the studied population, which represented only 0.4% of the total population, compared to the total number of new cases of leprosy detected in French Polynesia during the period of the study. The relative risk (RR = 30.8) of developing leprosy for contact individuals was surprisingly high in our study, as compared to other studies from highly endemic countries (RR generally around 4), as reviewed by Nordeen (18) . This may reflect intense case-finding activities in the contact population (5 indeterminate cases among the 14 patients), spontaneous healing of many paucibacillary cases in the noncontact population, or under reporting in this latter population. The occurrence of family clustering is well recognized, particularly in low-endemic areas. Despite the high burden of work involved, regular clinical follow up of family contacts should be considered in low-endemic areas in order to reach the goal of elimination of leprosy. The identification and treatment of early subclinical leprosy before it develops clinical manifestations may appear theoretically attractive, but the viability of such approaches in practical terms for disease control is open to question. The tracing of household contact individuals was motivated by epidemiological evidence of a higher risk of developing the disease among household contacts than in the general population (9), but in leprosy-endemic communities, it is known that the majority of cases arises among individuals without an obvious contact relationship with known cases (21).
The great magnitude of the task to collect and test periodically repeated specimens is to be pointed out. Despite these efforts, most of the individuals (64%) could not be retested, either because they did not feel concerned by the study or because they moved to another place. The serological monitoring implied a very large data-processing task to keep track of thousands of specimens and to ensure that source individuals could be identified in the future. Such arguments weigh heavily against the practicability of extensive serological screening for large-scale leprosy control programs. The cost-effectiveness equation should be most favorable in small communities with high incidence and ready access to sophisticated laboratory services, even though it is obvious that such a measure would be very difficult to manage under field conditions and not worthwhile in practice as proved by our data.
Future serological tests would be useful for the detection of the infection by M. leprae for both a better understanding of the dynamics of the infection and for the evaluation of the impact of control measures. However, for the objective of early diagnosis of leprosy among at-risk populations, these tests may not have operational usefulness in the context of a leprosy control program.
Acknowledgment. This research was supported by Institut Pasteur de Paris and Fondation Raoul Follcreau. We are grateful to Dr. P. Brennan for providing PGL-I antigen and antibody through funds from NIAID (contract no. 1 -AI-52582) and Dr. T. Fujiwara for NTP antigen.
REFERENCES
1. ABE, M., MINAGAWA, F., YOSHINO, Y., OZAWA, T., SAIKAWA, K. and SAITO, T. Fluorescent leprosy antibody absorption (FLA-ABS) test for detecting subclinical infection with Mycobacterium leprae. Int. J. Lepr. 48(1980)109-119.
2. BAGSHAWE, A. F., GARSIA, R. J., BAUMGART, K. and ASBURY, L. IgM serum antibodies to phenolic glycolipid I and clinical leprosy: two years' observation in a community with hyperendemic leprosy. Int. J. Lepr. 58(1990)25-30.
3. CARTEL, J.-L., BOUTIN, J. P., SPIEGEL, A., GLAZIOU, P., PLICHART, R., CARDINES, R. and GROSSET, J.-H. Leprosy in French Polynesia; epidemiological trends between 1946 and 1987. Lepr. Rev. 63(1992)211-222.
4. CARTEL, J.-L., CHANTEAU, S., BOUTIN, J. P., PLICHART, R., RICHEZ, P., ROUX, J. F. and GROSSET, J.-H . Assessment of antiphenolic glycolipid IgM levels using an ELISA test for detection of M. leprae infection in populations of the South Pacific Islands. Int. J. Lepr. 58(1990)512-517.
5. CHANTEAU, S., CARTEL, J.-L., CELERIER, P., PLICHART, R., DESFORGES, S. and Roux, J. F. PGL-I antigen and antibody detection in leprosy patients; evolution under chemotherapy. Int. J. Lepr. 57(1989)735-743.
6. CHANTEAU, S., CARTEL, J. L., GUIDI, C, PLICHART, R. and BACH, M. A. Seroepidemiological study on 723 household contacts of leprosy patients in French Polynesia using the disaccharide-Octyl-BSA as antigen. Int. J. Lepr. 55(1987)626-632.
7. CHO, S.-N., YANAGIHARA, D. L., HUNTER, S. W., GELBER, R. H. and BRENNAN, P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
8. DOUGLAS, J. T., CELONA, R. V., ABALOS, R. M., MADARANG, M. G. and FAJARDO, T. Serological reactivity and early detection of leprosy among contacts of lepromatous patients in Cebu, The Philippines. Int. J. Lepr. 55(1987)718-721.
9. FINE, P. E. M. Leprosy, the epidemiology of a slow bacterium. Epidemiol. Rev. 4(1982) 161-168.
10. FINE, P. E. M. Immunological tools in leprosy control. Int. J. Lepr. 57(1989)671-686.
11. FINE, P. E. M., PONNIGHAUS, J. M., BURGESS, P., CLARKSON, J. A. and DRAPER, C. Seroepidemiological studies of leprosy in northern Malawi based on an enzyme-linked immunosorbent assay using synthetic glycoconjugate antigen. Int. J. Lepr. 56(1988)243-254.
12. GODAL, T. and NEGASSI, K. Subclinical infection in leprosy. Br. Med. J. 3,(1973)557-559.
13. GROENEN, G., PATTYN, S. R., GHYS P., TSHILUMBA, K., KUYKENS, L., COLSTON, M. J. and YALISOMBO STUDY GROUP. A longitudinal study of the incidence of leprosy in a hyperendemic area in Zaire, with special reference to PGL-I antibody results. Int. J. Lepr. 58(1990)641-650.
14. HARBOE, M., CLOSS, O., BJUNE, G., KRONVALL, G. and AXELSEN, N . H . Mycobacterium leprae specific antibodies detected by radioimmunoassay. Seand. J. Immunol. 7,(1978)111-120.
15. HUNTER, S. W. and BRENNAN, P. J. A novel glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bactcriol. 147(1981)728-735.
16. MADDISON, S. E., STEWART, C. C, FARSHY, C. E. and REIMER, C. B. The relationship of race, sex and age to concentrations of serum immunoglobulins expressed in international units in healthy adults in the USA. Bull. WHO 52(1975)179-189.
17. NAVALKAR, R. G. Immunologic analysis of Mycobacterium leprae antigens by means of diffusion gel methods. Int. J. Lepr. 39,(1971)105-112.
18. NORDEEN, S. K. The epidemiology of leprosy. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1985, pp. 15-30.
19. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
20. SOEBONO, H. and KLATSER, P. R. A seroepidemiological study of leprosy in high- and low-endemic Indonesian villages. Int. J. Lepr. 59(1991)416-425.
21. TAYLOR, C. E., ELLISTON, E. P. and GIDEON, H. Asymptomatic infection in leprosy. Int. J. Lepr. 33(1965)716-727.
22. ULRICH, M., SMITH, P. G., SAMPSON, C, ZUNIGA, M., CENTENO, M., GARCIA, V., MANRIQUE, X., SALGADO, A. and CONVIT, J. IgM antibodies to native phenolic glycolipid-I in contacts of leprosy patients in Venezuela: epidemiological observations and a prospective study of the risk of leprosy. Int. J. Lepr. 59(1991)405-415.
23. Young, D. B. and BUCHANAN, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221,(1983)1057-1059.
1. Ph.D.; Institut de Recherches Medicales Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
2. M.D.; Institut de Recherches Medicales Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
3. Technician; Institut de Recherches Medicales Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
4. Technician; Institut de Recherches Medicales Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
5. Computer Scientist; Institut de Recherches Medicales Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
6. M.D.; Institut de Recherches Medicales Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
7. M.D., Institut de Recherches Medicales Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
Received for publication on 29 April 1993.
Accepted for publication in revised form on 19 August 1993.