- Volume 61 , Number 4
- Page: 556–62
HIV-1 Infection as a risk factor for leprosy; a case-control study in Tanzania
ABSTRACT
A case-control study was carried out in Tanzania to determine the relative risk of those with HIV-1 infection for getting leprosy. Cases were 93 consecutively diagnosed patients with leprosy aged 15-54 years f rom the Mwanza Region. Controls were a representative population sample of 4161 people drawn f rom a stratified cluster sample f rom urban areas, roadside settlements, and rural villages. HIV-1 infection was determined by enzyme-linked immunosorbent assay (ELISA); Western blot was used when the ELISA result was indeterminate. The HIV-1 prevalence in leprosy cases was 10% in rural (7 of 72) and in roadside and urban areas (2 of 21); in controls these prevalences were 3.4% and 9.9%, respectively. The relative risk of HIV-1 infection for the development of leprosy was estimated to be 2.2 [95% confidence interval (CI) = 1.0-4.7; p = 0.07]. HIV-1 infection was significantly associated with multibacillary (MB) leprosy (odds ratio 4.6; CI = 1.3-13.2) but not with paucibacillary leprosy (odds ratio 1.4; 95% CI = 0.4-3.8). The population etiological fraction for the development of MB leprosy attributable to HIV-1 infection in this population is estimated to be 13% (95% CI = 4%-23%). We conclude that HIV-1 is a risk factor for the development of MB leprosy. The impact of the HIV-1 epidemic on the incidence of leprosy so far has been limited since HIV-1 occurs mainly in urban areas and leprosy in rural areas.RÉSUMÉ
Une étude cas-témoins été réalisée en Tanzanie pour déterminer le risque relatif de développer la lèpre associé à une infection par le VIH-1. Les cas se composaient de 93 patients diagnostiques consécutivement comme ayant la lèpre, âgés de 15 à 54 ans et originaire de la Région de Mwanza. Les témoins étaient un échantillon représentatif de la population de 4161 personnes tirées au sort sur base d'un échantillonnage stratifié de zones urbaines, de zones établies situées le long des routes et de villages ruraux. L'infection à VIH-1 a été déterminée par un test immuno-enzymatique (ELISA); le Western-Blot a été utilisé en cas de résultat ELISA indéterminé. La prévalence de VIH-1 parmi les patients lépreux était de 10% en zones rurales (7 sur 72), ainsi que le long des routes et en zones urbaines (2 sur 21). Parmi les témoins, ces prévalences étaient respectivement de 3.4% et 9.9%. Le risque relatif de développer la lèpre associé à une infection à VIH-1 a été estimé à 2.2 [intervalle de confiance à 95% (IC) = 1.0 - 4.7; p = 0.07]. L'infection à VIH-1 était significativement associée à la lepre multibacillaire (MB) (odds ratio 4.6; IC = 1.3-13.2) mais pas avec la lèpre paucibacillaire (odds ratio 1.4; IC à 95% = 0.4-3.8). La fraction étiologique du risque de développer une lèpre MB attribuable à l'infection VIH-1 dans cette population est estimée à 13% (IC 95% = 4%-23%). Nous en concluons que le VIH-1 est un facteur de risque pour le développement de la lèpre MB. L'impact de l'épidémie à VIH-1 sur l'incidence de la lèpre a été limité jusqu'à présent du fait que l'infection à VIH-1 survient principalement en zones urbaines et la lèpre en zones rurales.RESUMEN
Se efectuó en Tanzania un estudio para determinar el riesgo relativo que tienen los casos con infección por H1V-1 para desarrollar lepra. Los casos estudiados fueron 93 individuos de la región de Mwanza cuyas edades fluctuaron entre loss 15 y los 54 años. Los controles fueron 4161 personas correspondientes a diferentes estratos poblacionales e incluyeron habitantes de áreas urbanas, habitantes de asentamientos al lado de carreteras, y habitantes de poblaciones rurales. La infección por HIV-1 se determinó por un inmunoensayo enzimático (ELISA); la inmunoelectrotransferencia (Western blot) se utilizó cuando los resultados por ELISA fueron inciertos. La prevalência de HIV-1 en los casos de lepra fue del 10% tanto en las áreas rurales (7 de 72) como en las áreas urbanas y en aquellos asentamientos al lado de carreteras (2 de 21 ); en los controles, estas prevalencias fueron de 3.4 y de 9.9, respectivamente. Se calculó que el riesgo relativo de la infección por HIV para el desarrollo de lepra fue de 2.2 [intervalo de confianza al 95% (CI) = 1.0-4.7; p = 0.07]. La infección por HIV-1 estuvo asociada significativamente con la lepra multibacilar (MB) (probabilidad 4.6; CI = 1.3-13.2) pero no con la lepra paucibacilar (probabilidad 1.4; CI = 0.4-3.8). La fracción de la población etiológica para el desarrollo de lepra MB atribuíble a la infección por HIV-1 en esta población fue de 13% (CI = 4%-23%). Se concluyó que el HIV-1 es un factor de riesgo para el desarrollo de lepra MB. La información relativa al impacto del HIV-1 epidémico sobre la incidencia de lepra es hasta ahora limitada debido a que HIV-1 oceurre principalmente en las áreas urbanas y la lepra en las áreas rurales.Although HIV-1 infection has been shown to be strongly associated with the development of active tuberculosis (4,5,10,16) and disease caused by other mycobacteria (1,12), its association with leprosy is much less clear. In a hospital-based case-control study in Zambia (11) an association was shown between HIV-1 infection and leprosy, but this was not confirmed in other hospitalbased studies in Ethiopia (17) and Yemen and various countries in Africa (8) nor in a community-based study in Malawi (13).
The Mwanza Region is situated on the shores of Lake Victoria in northwest Tanzania, and has a population of two million. In this region two studies were carried out around the same time: HIV-1 testing of leprosy and tuberculosis patients by the National Tuberculosis/Leprosy Program, as part of a collaborative study of the International Union Against Tuberculosis and Lung Disease, the World Health Organization, and the Tanzania National Tuberculosis and Leprosy Program (April-September 1991); and a population survey by the National Institute for Medical Research, as part of the Tanzania-Netherlands Research Project on AIDS and HIV infection, and AMREF's STD/HIV Intervention Project (August 1990-Fcbruary 1991). This provided the opportunity for a secondary data analysis in which data on leprosy cases were combined with data on population controls in a case-control study to determine the relative risk of HIV-1 infection for developing leprosy. A similar comparison between tuberculosis cases and population controls is reported separately (18).
METHODS
Cases. From April to September 1991 all 93 newly diagnosed cases of leprosy, aged 15-54 years from the Mwanza Region who were started on treatment, were enrolled in the study. Included were seven relapsed cases who had been declared cured in the past. Excluded were patients who had started treatment elsewhere and were transferred in while on treatment, patients not resident in the Mwanza Region, and patients returning to treatment after defaulting. The intake took place in all nine hospitals of the Mwanza Region.
Information was recorded on sex, age and residence. All patients were examined clinically by experienced field supervisors. The standard clinical examination forms were all scrutinized by an experienced Medical Officer (JvdB), who also examined all cases with an unclear classification. The Ridley-Jopling classification was used (6), with the modification that midborderline (BB) leprosy was classified as either borderline lepromatous (BL) or borderline tuberculoid (BT) leprosy. From each patient four slitskin smears were taken. The skin smears were examined locally and in the reference laboratory (Bugando Medical Center) by Ziehl-Neelsen staining and direct microscopy. Results were graded negative if no Mycobacterium leprae were seen in 100 fields, and positive if the bacterial index (BI) 61, was 1 + or more on the Ridley logarithmic scale (14,19), i.e., if at least one bacillus was detected in 100 fields examined. Skin biopsies were not taken routinely. Clinical and microbiological classification of leprosy cases occurred blind to HIV-1 status.
The definitions used in this study were (19,20,21): a) Multibacillary leprosy: a clinical presentation consistent with lepromatous (LL) or borderline lepromatous (BL) leprosy or at least one skin smear with a BI of 1 + or more, b), Paucibacillary leprosy: a clinical presentation of tuberculoid (TT) or borderline tuberculoid (BT) leprosy and a BI of 0.
A 5-10-ml sample of venous blood was collected from all cases as soon as possible after starting treatment for leprosy. HIV-1 antibodies were demonstrated by ELISA (Vironostika anti-HTLV-III; Organon Teknika, Boxtel, The Netherlands). On all samples with indeterminate ELISA results Western blot was performed (Novopath; Bio-Rad, Hercules, California, U.S.A.).
Controls. A full report on the population survey, which was carried out from August 1990 to February 1991, has been published elsewhere (2). For the selection of controls, the Mwanza Region was divided into three strata: urban, roadside, and rural. The urban stratum comprised Mwanza Municipality; the roadside stratum was made up of the small towns and villages along the main roads; and the rural stratum consisted of all other villages. From each stratum, 20 sites were randomly selected, with the probability of selection proportional to population size. Within selected sites, ten-cell units were selected using a simple random sampling to give an average total of 100 eligible individuals, aged 15-54 years, in each rural site and 50 eligible individuals in each roadside and urban site. (A ten-cell unit comprises, on the average, ten households.) Because of variability in the sizes of the tencell units, the final numbers selected for inclusion in the rural, roadside and urban strata were 2434, 1 157, and 1554, respectively. The number of study participants in the three strata were 2019, 958, and 1184, giving participation rates of 83%, 83%, and 76%, respectively. Variables recorded included age, sex, and residence. Venous blood (5-10 ml) was taken and separated in the field. Serum was tested for HIV-1 antibodies with ELISA (Organon Teknika). All nonnegative samples underwent confirmatory testing using Western blot (Organon; Epitope, Beaverton, Oregon, U.S.A.).
Data analysis. Cases and controls were subdivided by residential strata, sex, and age groups. Due to the small number of leprosy cases in the urban and roadside strata, these strata were combined in the analysis. In order to make the HIV-1 results between cases and controls comparable, HIV-1 status in both groups was based on the ELISA results; for those with an indeterminate ELISA result the Western blot result was used. The Western blot was considered positive if at least two of the gp41, gp120, and gp160 bands were present (22). Odds ratios (ORs) and their confidence intervals were calculated with the exact method (EGRET computer program), summarizing over subgroups after stratification by age, sex, and residence, and are used as estimates of relative risk. The population etiological fraction was estimated taking into account stratification of the sample by residence, age, and sex using the following formula (15):
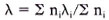
where λ is the population etiological fraction, ni is the number of cases in stratum i, and λi is the etiological fraction for stratum i. λi is calculated as:
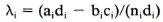
where ai is the number of exposed cases, di the number of unexposed controls, bi the number of exposed controls, ci the number of unexposed cases, and ni the number of cases in stratum i. Strata with 0 cases were ignored. The variance and confidence intervals were also calculated according to Schlesselman (15).
RESULTS
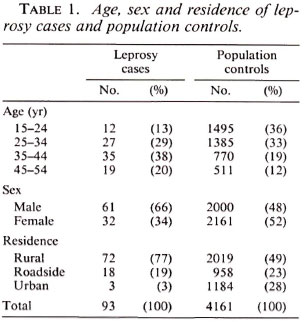
In the study period 93 cases of leprosy were identified. The distribution of age, sex and residence of cases and controls is presented in Table 1. Leprosy patients were, on the average, older than the controls and a higher proportion of them were males. The residence of the leprosy patients was more often the rural villages. The geographic distribution of the leprosy cases identified in this study is presented in The Figure.
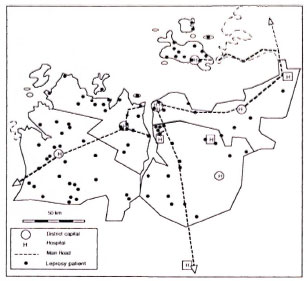
The figure. Map of Mwanza Region, Tanzania, indicating the residences of identified leprosy cases.
Of the 93 cases, 28 had multibacillary (MB) leprosy (LL 10, BL 18), and 65 had paucibacillary (PB) leprosy (BT 44, TT 21). All LL and 16 out of 18 BL cases had a BI of 1 + or more. All BT and TT cases had a BI of 0.
The prevalence of HIV-1 infection in MB and PB cases of leprosy and population controls by age, sex, and residence is presented in Table 2. HIV-1 prevalence was higher in MB than in PB cases. Overall, the HIV-1 prevalence in leprosy cases was 7 of 72 (10%) in rural, and 2 of 21 (10%) in roadside and urban areas; in controls these prevalences were 3.4% and 9.9% respectively. Of the 7 relapsed cases, 5 had MB leprosy, 1 of whom had HIV-1 infection; the 2 relapsed cases with PB leprosy were HIV-1 negative.
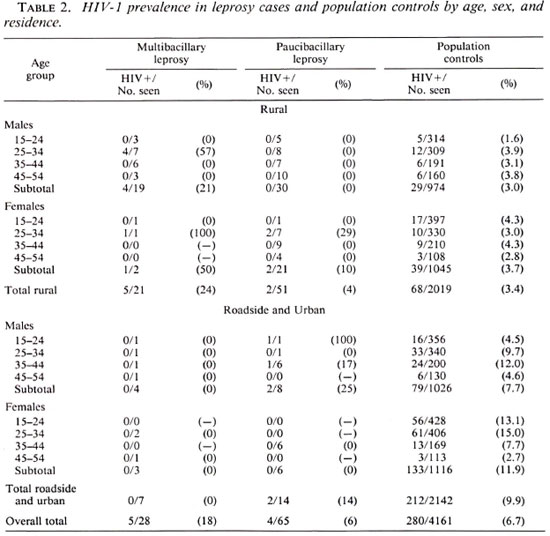
The overall odds ratio for the association between HIV-1 and leprosy that can be calculated from Table 2 was 2.2 (95% CI 1.0 - 4.7; p = 0.07). The odds ratio for the association between HIV-1 and MB leprosy was 4.6 (95% CI = 1.3-13.2; p = 0.02); for PB leprosy the odds ratio was 1.4 (95% CI = 0.4-4.7; p = 0.72). When relapsed cases are excluded from the analysis the odds ratio for the association between HIV-1 and MB leprosy was 4.1 (95% CI = 1.0-13.3; p = 0.06).
The population etiological fraction for the development of MB leprosy can be estimated to be 13% (95% CI = 4-23%). If the association between HIV-1 infection and MB leprosy were causal, 13% of MB leprosy cases occurring at present would be attributable to HIV-1 infection.
DISCUSSION
This population-based study has demonstrated an association between HIV-1 infection and the development of MB leprosy in self-reported cases. However, because the number of cases was small, this finding would need to be confirmed in other, if possible, larger studies. An association between HIV-1 infection and MB leprosy theoretically is to be expected, because a reduced cellular immunity, which occurs with the progress of HIV-1 infection, is associated with MB leprosy (7). It is also expected because of the association between HIV-1 infection and other diseases due to mycobacteria, in particular M. tuberculosis (1,4,5,10,12,16)
Comparing self-reported cases with population controls has a danger of introducing a bias, for instance, if health service utilization rather than the disease under study itself were associated with HIV-1 infection. As was done by Pönnighaus, et al. (13), we assessed the validity of the case-control comparison by applying the same methods to a comparison of tuberculosis cases and population controls. The odds ratio for the association between HIV-1 and tuberculosis was 8.3 (95% CI = 6.4-11.0) (18), which is consistent with findings of Ponnighaus, et al. (13) and others (4,5,10). This provides some support for the validity of the case-control comparison.
For the detection of leprosy cases use was made of passive case finding. Self-reported cases might be expected to show a bias toward more serious cases and toward a residence close to a hospital or a main road. The latter bias is not obvious (The Figure). On the contrary, most leprosy cases were resident in fairly remote rural villages. The clinical presentation and disability grade of HIV-1-infected patients was similar to that of patients without HIV-1 infection. However, because of the possibility of the former bias, our results can be interpreted in two ways: a) an increased incidence of MB leprosy may be attributable to HIV-1 infection or b) HIV-1 infection may contribute to a more serious clinical presentation of leprosy. The odds ratio for the association between MB leprosy and HIV-1 infection has not been influenced much by the inclusion of the relapsed cases.
Another limitation of this study may lie in the classification of leprosy disease. As has been shown elsewhere (3), the concordance between clinical and bacteriological classification was not complete. In particular, 2 of 28 (7%) cases clinically diagnosed as MB (both BL) did not have a positive slit-skin smear result. One of these two was HIV-1 infected. If these two cases were reclassified as PB leprosy, the odds ratio for the association between MB leprosy and HIV-1 infection would be 3.6 (95% CI = 0.9-11.6; p = 0.08). The classification of these two cases therefore had some influence on the results obtained. This provides an additional reason for carrying out further studies to confirm the association between HIV-1 and MB leprosy.
The association between HIV-1 infection and leprosy is only apparent in the rural area and not in the urban area or roadside settlements. This may be due to the small number of leprosy patients in the urban area and roadside settlements. The data from these strata have been included for completeness, although they do not contribute much to the final result.
The interaction of HIV-1 and leprosy is difficult to demonstrate because of a number of factors: a) leprosy occurs mainly in the rural areas, while HIV-1 is most prevalent in urban areas; b) the number of new cases of leprosy detected is small in most areas; c) the development of leprosy disease is a slow process, probably taking many years; and d) the loss of immunity in HIV-1 disease is also a slow process, usually taking a number of years to develop. Therefore, future case-control studies aiming at confirming or refuting the association between HIV-1 infection and an increased incidence of leprosy would need to: a) select "incident" cases of leprosy; b) control for confounders such as age, sex, and residence; c) be carried out in areas where leprosy is fairly common and HIV-1 infection has been well established in the rural areas for a number of years.
All of these criteria were met in the study by Pönnighaus, et al. in Malawi (13) with the exception that HIV-1 had probably been introduced recently in the study area. The studies by Leonard, et al. (8) and Tekle-Haimanot, et al. (17) appear to have had insufficient control for potential confounders, in particular age (8) and residence (17). The study of Leonard, et al. included some areas where HIV-1 infection had been introduced recently while this is unclear from the study of Tekle-Haimanot, et al. These factors might explain the negative findings of these studies.
The implications of our findings for leprosy control are worrying. In our study 4% to 23% of MB leprosy cases appear to be attributable to HIV-1 infection. If the HIV-1 prevalence increases in the rural areas of the region, the population etiological fraction is likely to increase as well. Over the past 10 years the case detection rate of MB leprosy has been stable, but the numbers are rather small. Hopefully an increase in the incidence of leprosy can be prevented by high cure rates through effective chemotherapy and case holding, by early case detection to limit the infectious period, and by BCG vaccination (9).
Repeating this study elsewhere and in 5 years time in the same location is recommended in order to assess the impact of HIV-1 infection on the epidemiology and control of leprosy.
Acknowledgment. We thank the Principal Secretary, Kenya Ministry of Health, and the Director-General of the National Institute for Medical Research for permission to carry out and publish the results of this study. We are grateful to the district medical officers, district tuberculosis and leprosy coordinators, and the laboratory staffof the hospitals in the Mwanza Region for their support. Data collection on leprosy cases was financed by the International Union Against Tuberculosis and Lung Diseases and the World Health Organization. The population survey study was financed by the Netherlands Minister for Development Cooperation and the AIDS Task Force of the European Community. We thank Prof. A. S. Muller, Dr. J. Velema, and Dr. W. Deville for critically reviewing the manuscript.
REFERENCES
1. AMERICAN THORACIC SOCIETY. Mycobacterioses and the acquired immune deficiency syndrome. Am. Rev. Respir. Dis. 136(1987)492-496.
2. BARONGO, L. R., BORGDORFF, M. W., MOSHA, F. F., NICOLL, A., GROSSKURTH. H., SENKORO, K. P., NEWELL, J. N., CHANGALUCHA, J., KLOKKE, A. H., KILLEWO, J. Z., et al. The epidemiology of HIV-1 infection in urban areas, roadside settlements and rural villages in Mwanza Region, Tanzania. AIDS 6(1992)1521-1528.
3. BECX-BLEUMINK, M. Allocation of patients to paucibacillary or multibacillary drug regimens for the treatment of leprosy: a comparison of methods based mainly on skin smears as opposed to clinical methods; Alternative clinical methods for classification of patients. Int. J. Lepr. 59(1991)292-303.
4. BRAUN, M. M., BADI, N., RYDER, R. W., BAENDE, E., MUKADI, Y., NSUAMI, M., MATELA, B., WILLIAME, J. C, KABOTO, M. and HEYWARD, W. A retrospective cohort study of the risk of tuberculosis among women of childbcaring age with HIV infection in Zaire. Am. Rev. Respir. Dis. 143(1991)501-504.
5. DE COCK, K. M., GNAORE, E., ADJORLOLO, G., BRAUN, M. M., LAFONTAINE, M. F., YESSO, G., BRETTON, G.. COULIBALY, I. M., GERSHY-DAMET, G. M., BRETTON, R., et al. Risk of tuberculosis in patients with HIV-I and HIV-II infections in Abidjan, Ivory Coast. BMJ 302(1991)496-499.
6. DHARMENDRA. Classifications of leprosy. In: Leprosy. Hastings, R. C , ed. Edinburgh: Churchill Livingstone, 1985, pp. 88-99.
7. HARHOE, M. The immunology of leprosy. In: Leprosy. Hastings, R. C , ed. Edinburgh: Churchill Livingstone, 1985, pp. 53-87.
8. LEONARD, G., SANGARE, A., VERDIER, M., SASSOU-GUESSEAU, E., PETIT, G., MILAN, J., M'BOUP, S., REY, J. L., DUMAS. J. L., HUGON, J., el at. Prevalence of HI V infection among patients with leprosy in African countries and Yemen. J. Acquir. Immune Dcfic. Syndr. 3(1990)1109-1113.
9. Lienhardt, C. and FINE, P. E. M. Controlling leprosy. BMJ 305(1992)206-207.
10. Long, R., Scalcini, M., Manfreda, J., Carre, G., Philippe, E., Hershfield, E., Sckla, L. and STACIIIW, W. Impact of human immunodeficiency virus type 1 on tuberculosis in rural Haiti. Am. Rev. Respir. Dis. 143(1991)69-73.
11. MEERAN, K. Prevalence of HIV infection among patients with leprosy and tuberculosis in rural Zambia. BMJ 298(1989)364-365.
12. PITCHENIK, A. E. The treatment and prevention of mycobacterial disease in patients with HIV infection. AIDS 2 Suppl. 1(1988)S177-S182.
13. PONNIGHAUS, J. M., MWANJASI, L. J., FINE, P. E. M., SHAW, M.-A., TURNER, A. C, OXDORROW, S. M., LUCAS, S. B., JENKINS, P. A., STERNE, J. A. C. and BLISS, L. Is HIV infection a risk factor for leprosy? Int. J. Lepr. 59(1992)221-228.
14. REES, R. J. W. The microbiology of leprosy. In: Leprosy. Hastings, R. C , cd. Edinburgh: Churchill Livingstone, 1985, pp. 31-52.
15. SCHLESSELMAN, J. J. Case-Control Studies-Design, Conduct. Analysis. Oxford: Oxford University Press, 1982, pp. 220-226.
16. SELWYN, P. A., HÄRTEL, D., LEWIS. V. A., SCHOENBAUM. E. E., VERMUND, S. H., KLEIN, R. S., WALKER, A. T.. and FRIEDLAND, G. H. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N. Engl. J. Med. 320(1989)545-550.
17. TEKLE-HAIMANOT, R., FROMMEL, D., TADESSE, T., VERDIER. M., ABEBE. M. and DENIS, F. A survey of HTLV-1 and HIVs in Ethiopian leprosy patients. AIDS 5(1991)108-110.
18. VAN DEN BROEK, J., BORGDORFF, M. W., PAKKER, N. G., et al. HIV-1 infection as a risk factor for the development of tuberculosis disease: a population-based case-control study in Tanzania. Int. J. Epidemiol. 1993 (in press).
19. WHO EXPERT COMMITTEE ON LEPROSY. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
20. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
21. WHO STUDY GROUP. Epidemiology of leprosy in relation to control. Geneva: World Health Organization, 1985. Tech. Rep. Ser. 716.
22. WORLD HEALTH ORGANIZATION. Acquired immune deficiency syndrome (AIDS) -proposed WHO criteria for interpreting results from Western blot assays for HIV-1, HIV-2, and HTLV-I/ HTLV-II. Wkly. Epidemiol. Ree. 65(1990)281-283.
1. M.D., National Institute for Medical Research, P.O. Box 1462, Mwanza, Tanzania and Royal Tropical Institute, Mauritskade 63, 1092 AD Amsterdam, The Netherlands.
2. M.D.; National Tuberculosis/Leprosy Program, P.O. Box 9083, Dar es Salaam, Tanzania.
3. M.D.; National Tuberculosis/Leprosy Program, P.O. Box 9083, Dar es Salaam, Tanzania.
4. M.D., National Tuberculosis/Leprosy Program, P.O. Box 9083, Dar es Salaam, Tanzania.
5. M.Sc, Bugando Medical Center, P.O. Box 1370, Mwanza, Tanzania.
6. M.D., National Institute for Medical Research, P.O. Box 1462, Mwanza, Tanzania.
7. Ph.D., African Medical and Research Foundation, P.O. Box 1482, Mwanza, Tanzania and London School of Hygiene and Tropical Medicine, Keppel Street, WC1E 7HT, London, U.K.
Reprint requests to Dr. Borgdorff in Mwanza.
Received for publication on 11 February 19.93;
Accepted for publication in revised form on 4 June 1993.