- Volume 61 , Number 4
- Page: 609–18
Differential production of interleukin 1 (IL-1), IL-6, tumor necrosis factor, and IL-1 receptor antagonist by human monocytes stimulated with Mycobacterium leprae and M. bovis BCG
ABSTRACT
Human blood monocytes cultured in various serum conditions were stimulated with Mycobacterium leprae or M. bovis BCG and their cytokine-inducing abilities were compared. BCG, either live or killed, induced production of interleukin 1 (IL-1), IL-6, tumor necrosis factor (TNF), and IL-1 receptor antagonist (IL-1 ra). Live BCG at a lower bacterial number was more potent than killed BCG in the induction of IL-6 and TNF. In contrast to BCG, killed M. leprae induced few cytokines except for IL-1ra. Similar results were obtained when monocytes were cultured in the presence of untreated or heat-inactivated fetal bovine serum (FBS). When FBS and human serum (HS) were compared and the effect of heat inactivation was investigated, monocytes in HS produced the most cytokines, then those in FBS, irrespective of heat inactivation, and those in heat-inactivated HS produced the least cytokines. There were no differences between live and killed M. leprae, and BCG were far more potent than M. leprae in all of our experimental conditions, indicating that the poor cytokine (IL-1, IL-6 and TNF) inducing ability of M. leprae was not due to their viability. Cytokine production was partially in parallel with the phagocytosis of the mycobacteria. These results suggest that M. leprae favor their infection by evoking little host reaction through the induction of only low levels of immunostimulatory or proinflammatory cytokines but a substantial amount of immunosuppressive cytokine.RÉSUMÉ
Des monocytes de sang humain cultivés dans du serum sous diverses conditions ont été stimulés par du Mycobacterium leprae ou du BCG de M. bovis et leur capacité à induire des cytokines a été comparée. Le BCG, vivant ou tué, provoquait la production d'interleukine 1 (IL-I), IL-6, de facteur nécrosant des tumeurs (FNT) cd d'antagoniste du récepteur d'IL-1 (IL-1ra). Le BCG vivant en quantité bactérienne moindre était plus puissant que le BCG tué dans l'induction d'IL-6 ct FNT. En contraste avec le BCG, le M. leprae tué induisait peu de cytokines, excepté l'IL-1ra. Des résultats semblables ont été obtenus par des monocytes cultivés en présence de serum bovin foetal (SBF) non traité ou inactiveé par la chaleur. Quand le SBF et le serum humain (SH) ont été comparés et que l'effet de l'inactivation par la chaleur a été examiné, les monocytes cultivés dans le serum humain produisaient le plus de cytokines, suivis par ceux cultivés dans le SBF, indépendamment de l'inactivation par la chaleur, et ceux cultivés dans le SH inactivé par la chaleur le moins de cytokines. II n'y avait pas de différence entre le M, leprae vivant et tué, et le BCG était beaucoup plus puissant que M. leprae dans toutes nos conditions expérimentales, indiquant que la faible capacité de M. leprae à induire des cytokines (IL-1, IL-6, et FNT) n'était pas due à sa viabilité. La production de cytokine était partiellement en paralèlle avec la phagocytose des mycobactéries. Ces résultats suggèrent que M. leprae favoris son infection en suscitant peu de réaction de l'hôte par l'induction de faibles taux de cytokines stimulant l'immunité ou l'inflammation, mais un taux substantiel de cytokine immunosuppressive.RESUMEN
Se investigó la producción de citocinas por los monocitos de sangre periférica mantenidos bajo diversas condiciones de cultivo en respuesta a la estimulación con Mycobacterium leprae o con M. bovis. El BCG, vivo o muerto, indujo la producción de interleucina-1 (IL-1), IL-6, factor necrosante de tumores (TNF). y el antagonista del receptor para la IL-1 (IL-1ra). El BCG vivo fue más potente que el BCG muerto en la inducción de IL-6 y TNF. En contraste con el BCG, el M. leprae muerto sólo indujo la producción de algunas citocinas, entre ellas el IL-Ira. Se obtuvieron los mismos resultados cuando los monocitos se cultivaron en presencia de suero fetal de bovino (SFB) fresco o inactivado por calor. Cuando se compararon el SFB y el suero humano (SH) y se investigó el efecto de su inactivación por calor, se encontró que los monocitos en SH produjeron más citocinas que las producidas por los MN en SFB, independientemente de si el SFB estuvo o no inactivado por calor. Los MN en SH inactivado por calor produjeron la menor cantidad de citocinas. No hubo diferencia entre el M, leprae vivo y el M. leprae muerto por calor; el BCG fue ms potente que el M. leprae bajo todas las condiciones ensayadas, indicando que la pobre capacidad inductora de citocinas (IL-1, IL-6 y TNF) del M. leprae no estuvo relacionada con su viabilidad. La producción de citocinas estuvo parcialmente en paralelo con la fagocitosis de micobacterias. Estos resultados sugieren que M. leprae favorece su implantación por evocar una mínima reacción del huésped, induciendo en él la producción de bajos niveles de citocinas inmunoestimulatorias o proeinflamatorias y una cantidad substancial de citocinas inmunosupresoras.Leprosy and tuberculosis, caused by infection with Mycobacterium leprae and M. tuberculosis, respectively, are chronic infectious diseases from which more than 20 million patients are suffering worldwide (9,24). Although these two mycobacteria are common in several of their constituents, such as peptidoglycan and the arabinogalactanmycolic acid complex (1,23), the clinical aspects of the two diseases are complicated. Based on the clinical features as well as immunologic responses, leprosy patients can be grouped into four types or categories: lepromatous (L), tuberculoid (T), borderline (B) and indeterminate (I). Patients with tuberculoid leprosy manifest a strong cell-mediated immunity (CMI) response to M. leprae but produce a relatively low level of antibody. In contrast, in lepromatous patients there is a prominent humoral antibody response but an anergy in CM I to M. leprae (10).
Previous studies have suggested that these immunological deviations result from the accumulation of a particular subset of T cells in the lesions, namely, tuberculoid lesions contain a predominance of CD4+ T cells whereas lepromatous lesions contain mainly CD8+ T cells (19). It also has been reported that in tuberculoid lesions interleukin 2 (IL-2) and interferon-gamma (IFN-γ) mRNA, products of the Thl-type helper T cells, were being expressed while in lepromatous lesions IL-4, IL-5, and IL-10 mRNA, products of the Th2-type helper T cells, were expressed (31). Immunological dysfunctions of macrophages also have been reported. Macrophages of lepromatous patients suppress T-cell responses and produce minor or only low levels of IL-1 and tumor necrosis factor (TNF) (2,21,30). Since macrophages function as antigen-presenting cells to T cells and produce a variety of immunoregulatory cytokines, it is possible that the primary interaction between macrophages and mycobacteria results in the immunological deviation.
IL-1, IL-6 and TNF are major cytokines produced by macrophages, and these cytokines stimulate immunological and inflammatory reactions (4). In contrast, IL-1 receptor antagonist (IL-1ra), which is also produced by macrophages, inhibits IL-1 activity as well as IL-1-triggered chain reactions by competitively binding to the IL-1 receptors (5). IL-10 also inhibits macrophage functions and influences the subsequent macrophage/T-cell interaction (7). Therefore, macrophage function is regulated by both immunostimulative and immunosuppressive cytokines.
In this paper, we studied the production of cytokines, IL-1, IL-6, TNF and IL-1ra, by human monocytes stimulated with live or killed M. leprae or BCG, and showed that M. leprae is a very poor inducer of immunostimulatory cytokines compared to BCG. However, a substantial amount of IL-1ra can be induced by stimulation with M. leprae even when no other cytokines are induced. In addition, the phagocytosis of M. leprae and cytokine production appeared to depend partially on serum factor(s).
MATERIALS AND METHODS
Reagent. Human recombinant IL-1 α (2 x 107 U/ml) was provided by Dr. M. Yamada, Dainippon Pharmaceutical Co., Osaka, Japan, and human recombinant IL-2 by Shionogi Co., Osaka. Concentrated buffy coat from healthy donors was supplied by Aichi Red Cross Blood Center, Aichi, Japan. RPMI 1640 and polymyxin B were purchased from Sigma Chemical Co., St. Louis, Missouri, U.S.A., and FBS was obtained from Bocknek, Toronto, Canada.
Mycobacteria. M. leprae strain Thai-53 were grown in the foot pads of nude mice (16). Mouse foot pads were aseptically removed, minced with scissors, and homogenized with 7H12 medium. After centrifuging the homogenate for 10 min at 100 x g, the supernatants were obtained and again centrifuged for 20 min at 3500 x g. The precipitates were resuspended with 7H12 medium, and the bacillary number was determined by the method of Shepard and McRae (27). The bacillary number was consistent with that counted under microscopy with a hematocytometer. Freeze-dried M. bovis BCG were obtained from Japan BCG Company, Tokyo, Japan. The BCG were suspended with phosphate buffered saline (PBS). These mycobacteria were homogenized by mild sonication. The bacillary number was counted under microscopy with a hematocytometer. Heat-killed mycobacteria were obtained by treating them at 120ºC for 15 min.
Supernatants of monocytes stimulated with mycobacteria. The buffy coat from healthy donors was diluted 1:3 in Hanks' balanced salt solution (HBBS). Mononuclear cells (MNC) were separated over Ficoll-Hypaque, washed twice in HBBS, and suspended in RPMI 1640 medium supplemented with 100 U/ml of penicillin G, 100 µg/ml of streptomycin and 15 raM HEPES. The number of monocytes was estimated by incubating the cell suspension in a he matocytometer at 37ºC for 3 min in air containing 5% CO2 and then counting the spreading cells. The spreading cell number was consistent with that of cells adhering to the tissue culture plate. One ml of a MNC suspension containing monocytes (1 x 106 cells/ml) was added to each well of a 24-well plate (Falcon, Lincoln, New Jersey, U.S.A.). After 2 hr of culturing at 37ºC in air containing 5% CO2, the cells were washed twice with HBBS. More than 90% of the adherent cells were monocytes as determined by morphological criteria with Giemsa staining and the ability to phagocytose latex beads. To the adherent monocytes, 1 ml of RPMI 1640 supplemented with 1% fetal bovine serum (FBS) or human serum (HS), untreated or heat inactivated at 56ºC for 30 min, containing mycobacteria were added, and then the cells were cultured at 37ºC. Although the medium was endotoxin-free according to the Limulus amebocyte assay (sensitivity limit of 0.1 ng/ml), we usually added polymyxin B (5 µg/ml) to the culture to inhibit the effect of a small amount of endotoxin. After each culture period, the supernatants were obtained by centrifugation. These antibiotics (penicillin G, streptomycin and polymyxin B) did not affect the viability of M. leprae or BCG for 1 week or 24 hr, respectively, as determined by the metabolism of 14C-palmitic acid (8).
Assay for IL-1 activity. IL-1 activity was determined by a proliferation assay with an IL-1-dependent mouse T-cell line, D1ON4M, which was provided by Dr. S. J. Hopkins, University of Manchester, Manchester, U.K.(12). In brief, cells were cultured in RPMI 1640, 10 mM HEPES, antibiotics, 5 x 10-5 M 2-mercaptoethanoI, 10% FBS, concanavalin A (3 µg/mi), IL-2 (40 U/ml) and standard IL-1α or test samples. Cells (1 x 104) were cultured in wells of flat-bottom microtiter plates at 37ºC in 5% CO2 in air. After 3 days of culture, cell proliferation activity was assessed by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide or thiazolyl blue (MTT) method. After solubilization of the formozan with 20% SDS and 50% DMF (dimethyl formamide) in water, the absorbance at 595 nm was measured on an ELISA autoreader (Bio-Rad). IL-1 activity was expressed as unit equivalent to standard recombinant IL-lα.
Assay for IL-6 activity. The biological activity of IL-6 was measured by its proliferative action on the IL-6-dependent murine hybridoma clone MH60.BSF2 (provided by Dr. T. Hirano, Osaka University, Osaka, Japan) (15). Proliferation was measured by the MTT method (20). One unit of IL-6 activity was defined as the reciprocal of the dilution of samples that exhibited 50% of maximum response.
Assay for TNF activity. The activity of TNF was determined by a L929 fibroblast cell lytie assay (27). Briefly, 100 µl of a suspension of TNF-sensitive mouse L929 fibroblast cells (5 x 105 cells/ml) was cultured with serially diluted test samples in wells of a flat-bottom microtiter plate at 37ºC for 18 hr in air containing 5% CO2 in the presence of actinomycin D (1µg/ml). After culture, the plates were washed, and cell lysis was determined by staining the plates with crystal violet (0.5%) in methanol-water (1:25, v/v). After the dye-stained cells were solubilized with 0.1 ml of 0.1% SDS, the dye uptake was calculated by an ELISA autoreader. One unit of TNF activity was defined as the reciprocal of the dilution of samples that lysed 50% of the L929 cells.
Determination of IL-1ra. IL-1ra content was determined by an ELISA using mouse monoclonal antibody (IgG) and rabbit polyclonal antibody (IgG) against human recombinant IL-1ra.
Phagocytosis by monocytes of M. leprae and BCG. Monocytes (5 x 106 cells) were cultured in RPMI 1640 medium supplemented with 1% untreated or heat-inactivated FBS or HS with killed M. leprae or killed BCG (1.5 x 107) on coverslips in 24-well culture plates. After culture for 24 hr, the coverslips were washed and stained with acid-fast staining. Phagocytosis of the mycobacteria by monocytes was determined under microscopy.
RESULTS
Cytokine production by monocytes stimulated with M. leprae and BCG. In order to determine the difference of cytokine production by monocytes stimulated with M. leprae and BCG, human monocytes (1 x 106 cells) were treated with varying numbers of killed M. leprae, live or killed BCG. Because cytokine induction by a human monocytic cell line with lipopolysaccharide requires at least 1% FBS (17), in this study we first conducted the experiment in the presence of 1% heat-inactivated FBS. TNF was determined after 6 hr of culture and IL-1, IL-6, and IL-1ra were determined after 24 hr of culture. As shown in Figure 1, up to 3 x 106 of M. leprae induced no detectable IL-1, IL-6 or TNF. At 1 x 107 they induced only very low levels of these three cytokines. In contrast, BCG, either live or killed, induced these cytokines at more than 3 x 104 or 105 cells; as shown 105-106 was the optimal. Live BCG induced larger amounts of cytokines than killed BCG at lower numbers of bacteria. Thus, BCG appeared to be far more potent than M. leprae. In contrast to IL-1, IL-6 and TNF, IL-1ra was induced by M. leprae-stimulated monocytes in a dose-dependent manner. BCG also induced IL-1ra, but its production was inversely related to the number of bacteria. Although the data are not shown, 104 live or killed BCG induced more IL-1ra than 105 BCG. A control culture with polymyxin B alone did not induce detectable levels of any of the cytokines.
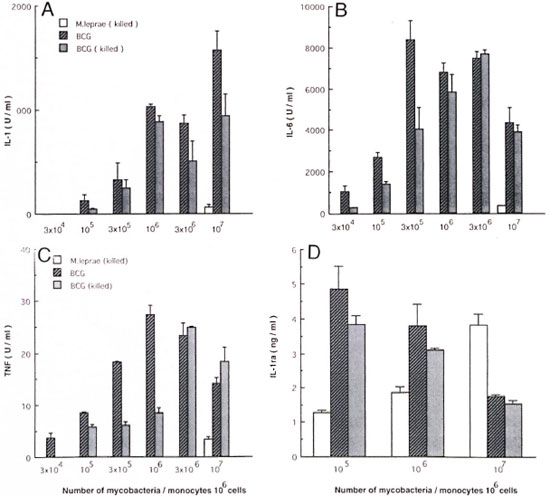
Fig. 1. Dose response of cytokine production by M. leprae- or BCG-stimulated monocytes. Human monocytes (106 cells) were cultured in medium supplemented with 1% heat-inactivated fetal bovine serum with varying numbers of killed M. leprae, live or killed BCG. After culture for 24 hr, amounts of IL-1, 1L-6, and IL-1ra in culture supernatants were determined as described in Materials and Methods. Amount of tumor necrosis factor (TNF ) was determined after a 6-hr culture. Mean ± of S.D. of triplicate cultures is shown. A = IL-1; B = IL6; C - TNF; D = IL-1ra.
Kinetics of cytokine production by monocytes. The differences in cytokine production might have resulted from different kinetics. Therefore, monocytes were stimulated with 107 mycobacteria, and kinetics studies of cytokine production were conducted. IL-1 and IL-6 production by monocytes stimulated with cither BCG or M. leprae increased with the duration of the incubation period and peaked at 18-24 hr. TNF production by BCG-stimulated monocytes peaked at 12-18 hr. M. leprae continuously induced IL-1ra up to 44 hr, but BCG did so only up to 12.5 hr. Therefore, the different cytokine production by monocytes stimulated with M. leprae and BCG did not appear to result from different kinetics.
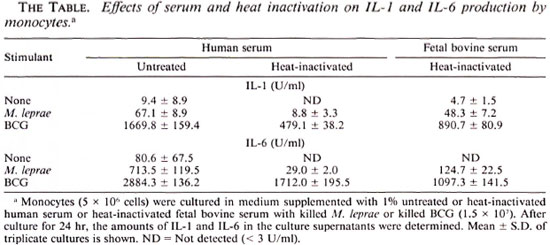
FBS and HS and the effect of heat-inaetivation. The experiments in Figures 1 and 2 were conducted in the presence of heatinactivated FBS. Since it is reported that phagocytosis of M. leprae by human monocytes depends on complement C3 (25), we determined the effect of heat-inactivation of the serum on cytokine production by monocytes stimulated with killed M. leprae or BCG. A comparison of FBS and HS was also made. As demonstrated in The Table, monocytes in untreated HS produced the highest levels of cytokines in response to either mycobacteria. Heat-inactivated FBS exhibited a lower effect. Although the data are not shown, there was no difference between untreated and heat-inactivated FBS. The monocytes in heat-inactivated HS produced the lowest levels of cytokine. In these experiments, however, BCG was again more potent than M. leprae.
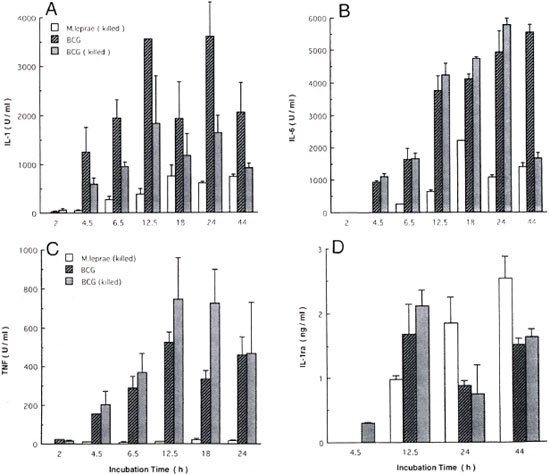
Fig. 2. Kinetics of cytokine production by monocytes. Monocytes (10'' cells) were cultured in medium supplemented with 1% heat-inactivated fetal bovine serum with killed M. leprae, live or killed BCG (107) for periods indicated. After culture, amounts of 1L-1, IL-6, tumor necrosis factor (TNF ) and IL-1ra in culture supernatants were determined. Mean ± S.D. of triplicate cultures is shown. A = IL-1; B = IL-6; C = TNF; D = IL-1ra.
Effect of serum on phagocytosis of mycobacteria by monocytes. In order to determine the effect of serum on the phagocytosis of these mycobacteria by monocytes, the monocytes were cultured in medium supplemented with untreated or heat-inactivated FBS or HS with killed M. leprae or killed BCG. Monocytes in untreated HS were most active in the phagocytosis of M. leprae. Monocytes in other cultures exhibited lower levels of phagocytosis. There was no difference between heat-inactivated HS, untreated and heat-inactivated FBS. In contrast to M. leprae, there was no difference in the phagocytosis of BCG between untreated and heat-inactivated HS. Monocytes in FBS either untreated or heat-inactivated exhibited lower levels of phagocytosis (data not shown). However, under the same experimental conditions, BCG were usually phagocytosed more than M. leprae.
Live and killed M. leprae in induction of cytokines by monocytes. Untreated HS appeared to be the most efficient constituent of the medium for monocytes to produce cytokines in response to mycobacteria. The monocytes then were cultured in the presence of untreated HS, and their ability to produce cytokines in response to live or killed M. leprae and live BCG was determined. As shown in Figure 3, significant but low levels of IL-1 and IL-6 were induced by a small number of M. leprae. No difference was observed between live or killed M. leprae. In contrast to IL-1 and IL-6, TNF was not induced by up to 106 M. leprae. BCG was more potent than M. leprae. IL-1ra was equally produced by 105 to 107 M. leprae. Again, no difference was observed between live and killed M. leprae. In contrast to the results in FBS, BCG induced IL-1ra in a dose-dependent manner.
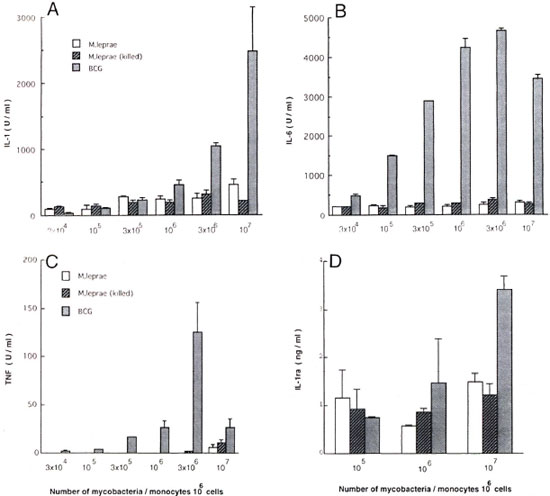
Fig. 3. Comparison of live and killed M. leprae in induction of cytokines. Monocytes (106 cells) were cultured in medium supplemented with 1% untreated human serum with live or killed M. leprae or live BCG (107). After culture for 24 hr, amounts of IL-1, IL-6 and IL-1ra in culture supernatants were determined. Amount of tumor necrosis factor (TNF ) was determined after a 6-hr culture. Mean ± S.D. of triplicate cultures is shown. A = 1L 1; B = IL-6; C = TNF; D = IL-1ra.
DISCUSSION
In this study, we demonstrated the difference between M. leprae and BCG in regard to their ability to induce cytokine production from human monocytes. The effects of live and killed mycobacteria and those of heat-inactivation of serum were also determined. BCG has been used as a vaccine for almost 50 years. Macrophages from mice administered BCG became tumoricidal and those from a normal mouse produced IL-6 in response to BCG in vitro (14,22). Therefore, it is expected that BCG is a potent inducer of cytokines from animal and human macrophages. The components of M. tuberculosis muramyl dipeptide (MDP), lipoarabinomannan (LAM), and proteins have been shown to induce cytokine production by human monocytes or macrophages (1,23,29). M. tuberculosis also is reported to induce IL-1 and TNF by human monocytes (29). However, as far as we know it is not clear what kind of cytokines BCG induce in human monocytes and that if they do, how BCG differs from virulent M. tuberculosis. In addition, an immunosuppressive cytokine IL-1ra has not been investigated in relation to its production by macrophages/monocytes stimulated with mycobacteria. In this study BCG, live or killed, induced production of IL-1, TNF, IL-6 and IL-1ra by human monocytes. Therefore, BCG, similar to M. tuberculosis, appeared to be a potent inducer of cytokines. IL-1, IL-6 and TNF were induced in a dose-dependent manner at small bacterial numbers and decreased at higher numbers. However, IL-1ra production was inversely related to the bacillary number, suggesting that a very small number of BCG can induce IL-1ra even when other cytokines are not induced. It is known that immunological tolerance can be induced by repeated injection of a very small amount or a very large amount of antigens (13). It is possible, therefore, that IL-1ra, an immunosuppressive cytokine, may play a role in the low-dose antigen-induced tolerance. IL-1ra production is induced by IL-4, granulocyte monocyte-colony stimulating factor, T-cell growth factor-β, and IL-10 (5,28). However, because IL-1ra decreased with the increase of the other cytokines, there may be a cytokine capable of inhibiting IL-1ra production.
Studies revealed that killed M. tuberculosis or BCG protected animals from tuberculosis only weakly, but live BCG did so for a long period (9). The same was true with other bacteria. Mouse macrophages stimulated with live Listeria monocytogenes produced much more IL-1 than those stimulated with killed bacteria (18). In our study, at low bacillary numbers live BCG was more effective than killed BCG in the induction of IL-6 and TNF, but not of IL-1 or IL-1ra. At high cell numbers, however, there was no difference. Therefore, the differential protective effect between live and killed BCG could not be explained by IL-1 induction.
When compared with BCG, M. leprae induced only small amounts of IL-1, IL-6 and TNF. A kinetic study indicated that the difference had not resulted from the different kinetics of cytokine production. It was of note, however, that IL-1ra was induced in a dose-dependent manner. The different dose-dependent responses between BCG and M. leprae presumably were due to the different efficacies of these mycobacteria. Thus, the finding indicates that IL-1ra is the most readily inducible cytokine by these mycobacteria.
It is reported that complement C3 binds to phenolic glycolipid-I in M. leprae, thereby facilitating phagocytosis of M. leprae by monocytes through complement receptors (25). Therefore, we examined the effect of heat inactivation of HS and FBS on phagocytosis and cytokine production. Indeed, monocytes in fresh HS phagocytosed M. leprae more than those in heat-inactivated HS or FBS. In contrast to M. leprae, there was no difference in the phagocytosis of BCG between untreated and heat-inactivated HS, indicating that complement is not involved. Monocytes in FBS, untreated or heat-inactivated, exhibited the smallest level of phagocytosis. It was of note, however, that under any experimental condition BCG was phagocytosed more than M. leprae. In parallel to the phagocytosis, monocytes in fresh HS produced more cytokines than those in heat-inactivated HS. However, when we compared HS and FBS the cytokine production was not in parallel because monocytes in heat-inactivated HS and FBS phagocytosed M. leprae at the same level. Therefore, phagocytosis is not the sole factor responsible for cytokine induction. It is also interesting that HS is more efficient than heat-inactivated HS in the case of BCG. Since there was no difference between the phagocytosis by these monocytes, heat-labile serum components may augment the capacity of monocytes in cytokine production.
When we compared live and killed M. leprae there was no difference. In addition, M. leprae appeared to be a very poor inducer of immunostimulatory cytokines IL1, IL-6 and TNF, while BCG stimulated much cytokine production under any experimental condition. In contrast, IL-1ra (an immunosuppressive cytokine) was induced by both mycobacteria. Since macrophage function is determined by a balance between stimulatory and suppressive cytokines, M. leprae appeared to confer immunosuppressive effects rather than immunostimulant ones. BCG induces local inflammation and systemic immunity against M. tuberculosis (9). However, it is not known whether primary infection of M. leprae induces any acute symptoms. Therefore, the potent cytokine-inducing ability of BCG was implicated in their immunostimulatory effect. In contrast, this study suggests that M. leprae escape from host defenses by inducing the least level of immunostimulatory or proinflammatory cytokine production but inducing substantial amounts of the immunosuppressive cytokine IL-1ra. Macrophages from patients with leprosy are reported to be defective in their ability to present M. leprae antigens to sensitized T cells (9,21) . Since IL-1 and IL-6 play important roles in antigen presentation, not only the production of low levels of IL-1 and IL-6 but also the production of substantial amounts of IL-1 ra are implicated in the immunodysfunction. The low level production of TNF further favors the infection and multiplication of M. leprae in monocytes because TNF enhances the production of reactive nitrogen oxide by murine macrophages and inhibits mycobacterial growth in murine and human macrophages (3,6). In this regard it may be interesting to investigate cytokine production by patients' monocytes. The cells may produce more IL-1ra and less IL-1, IL-6 and TNF in response to M. leprae than those of healthy individuals. It might also be interesting to investigate the component of M. leprae which is responsible for IL-1ra induction. Through genetic engineering the IL-1ra-inducing component could be deleted from M. leprae. Such mutated M. leprae may be able to induce more cytokines, thus becoming a good vaccine.
Acknowledgment. We thank Dr. S. Hida for the IL-Ira ELISA. This work was supported in part by a grant from the U.S.-Japan Cooperative Medical Science Program. The authors also wish to gratefully acknowledge the Sasakawa Memorial Health Foundation for their financial support of this study.
REFERENCES
1. BARNES, P. F., CHATTERJEE, D., ABRAMS, J. S., LU, S., WANG, E., YAMAMURA, M., BRENNAN, P. J. and MODLIN, R. L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. J. Immunol. 149(1992)541-547.
2. BARNES, P. F., CHATTERJEE, D., BRENNAN, P. J., REA, T. H. and MODLIN, R. L Tumor necrosis factor production in patients with leprosy. Infect. Immun. 60(1992)1441-1446.
3. BERMUDEZ, L. E. M. and YOUNG, L. S. Tumor necrosis factor, alone or in combination with IL2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J. Immunol. 140(1988)3006-3013.
4. DINARELLO, C. A. Interleukin-1 and its biologically related cytokines. Adv. Immunol. 44(1989)153-205.
5. DINARELLO, C. A. and THOMPSON, R. C. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol. Today 12(1991)404-410.
6. DING, A. H., NATHAN, C. F. and STUEHR, D. L. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141(1988)2407-2412.
7. FIORENTINO, D. F., ZLOTNIK, A., VIEIRA, O., MOSMANN, T. R., HOWARD, M., MOORE, K. W. and O'GARRA, A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th 1 cells. J. Immunol. 146(1991)3444-3451.
8. FRANZBLAU, S. G., WHITE, K. E. and O'SULLIVAN, J. F. Structure-activity relationships of tetramethylpiperidine-substituted phenazines against Mycobacterium leprae in vitro. Antimicrob. Agents Chemother. 33(1989)2004-2005.
9. FREUND, J. and MIDDLEBROOK, G. Tuberculosis. In: Bacteriology and Mycotic Infection. Dubos, R. J.,cd. Philadelphia: Lippincott Co., 1948, pp. 303-320.
10. HASTINGS, R. C, GILLIS, T. P., KRAHENBUHL, J. L. and FRANZBLAU, S. G. Leprosy. Clin. Microbiol. Rev. 1(1988)330-348.
11. HIRSCHBERG, H. The role of macrophages in the lymphoproliferativc response to M. leprae in vitro. Clin. Expl. Immunol. 34(1978)46-51.
12. HOPKINS, S. J. and HUMPHREYS, M. Simple, sensitive and specific bioassay for interleukin-1. J. Immunol. Methods 120(1989)271-276.
13. HOWARD, T. G. and MITCHISON, N. A. Immunological tolerance. Prog. Allergy 18(1975)43-96.
14. HUYGEN, K., VANDENBUSSCHE, P. and HEREMANS, H. Interleukin-6 production in Mycobacterium bovis BCG-injecled mice. Cell. Immunol. 137(1991)224-231.
15. KAWANO, M., HIRANO, T., MATSUDA, T., TAGA, T., HORI, Y., IWATO, K., ASAOKU, H., TANG, B., TANABE, O., TANABE, H., KURAMOTO, A. and KISNIMOTO, T. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature 332(1988)83-85.
16. MATSUOKA, M., KOIISAKA, K. and DAYANGHIRANG, A. J. Characterization of M. leprae Thai-53 strain. (Abstract) Int. J. Lepr. 60(1992)722.
17. MATSUSHIMA, K., COPELAND, T. D., ONOZAKI, K. and OPPENIIEIM, J. J. Purification and biochemical characteristics of two distinet human interleukins 1 from the myelomonocytic THP-1 cells. Biochemistry 3(1986)3424-4329.
18. MITSUYAMA, M., IGARASHI, K., KAWAMURA, I., OHMORI, I. and NOMOTO, K. Difference in the induction of macrophage interleukin-1 production between viable and killed cells of Listeria monocytogenes. Infect. Immun. 58(1990)1254-1260.
19. MODLIN, R. L., MELANCON-KAPLAN, J., YOUNG, S. M. M., PIRMEZ, C, CONVIT, J., REA, T. H. and BLOOM, B. R. Learning from lesions: patterns of tissue inflammation in leprosy. Proc. Natl. Acad. Sci. U.S.A. 85(1988)1213-1217.
20. MOSSMANN, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity. J. Immunol. Methods 65(1983)55-63.
21. NATH, I., VAN ROOD, R. R., MEHRA, N. K. and VAIDYA, M. C. Natural suppressor cells in human leprosy: the role of HLA-D-identical peripheral lymphocytes and macrophages in the in vitro modulation of lymphoproliferative responses. Clin. Exp. Immunol. 42(1980)203-210.
22. RUCO, L. P. and MELTZER, M. S. Defective tumoricidal capacity of macrophages from C3H/HcJ mice. J. Immunol. 120(1978)329-334.
23. SANCEAU, J., FALCOFF, R., BERANGER, F, CARTER, D. B. and WIETZERBIN, J. Secretion of interleukin-6(1L-6) by human monocytes stimulated by muramyl dipeptide and tumor necrosis factor alpha. Immunology 69(1990)52-56.
24. SANSARRICQ, H. Leprosy in the world today. Lepr. Rev. 52(1981)15-31.
25. SCHLESINGER, L. S. and HORWITZ, M. A. Phenolic glycolipid-1 of Mycobacterium leprae binds complement C3 in serum and mediates phagocytosis by human monocytes. J. Exp. Med. 174(1991)1031-1038.
26. SHEPARD, C. C. and MCRAE, D. H. A method for counting acid-fast bacteria. Int.J. Lepr. 36(1967)78-82.
27. TAMATANI, T., KIMURA, S., HASHIMOTO, T. and ONOZAKI, K. Purification of guinea pig tumor necrosis factor(TNF): comparison of its antiproliferative and difTerentiative activities for myeloid leukemic cell line with those of recombinant human TNF. J. Biochcm. 105(1989)55-60.
28. VANNIER, E., MILLER, L. C. and DINARELLO, C. A. Coordinated antiinflammatory effects of inlerleukin-4: interleukin 4 suppresses interleukin 1 production but up-regulates gene expression and synthesis of interleukin 1 receptor antagonist. Proc. Natl. Acad. Sci. U.S.A. 89(1992)4076-4080.
29. WALLIS, R. S., AMIR, T. M. and ELLNER, J. J. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins. Proc. Natl. Acad. Sci. U.S.A. 87(1990)3348-3352.
30. WATSON, S., BULLOCK W., NELSON, K., SCIIAUF, V., GELBER, R. and JACOBSON, R. Interleukin 1 production by peripheral blood mononuclear cells from leprosy patients. Infect. Immun. 45(1984)787-789.
31. YAMAMURA, M., UYEMURA, K., DEANS, R. J., WEINBERG, K., REA, T. H., BLOOM, B. R. and MODLIN, R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254(1991)277-279.
1. M.Sc; Department of Hygienic Chemistry, Faculty of Pharmaceutical Sciences. Nagoya City University, Mizuho, Nagoya 467, Japan.
2. U.S.; Department of Hygienic Chemistry, Faculty of Pharmaceutical Sciences. Nagoya City University, Mizuho, Nagoya 467, Japan.
3. Ph.D. Assistant Professor; Department of Hygienic Chemistry, Faculty of Pharmaceutical Sciences. Nagoya City University, Mizuho, Nagoya 467, Japan.
4. M.Sc, Assistant Professor; Department of Hygienic Chemistry, Faculty of Pharmaceutical Sciences. Nagoya City University, Mizuho, Nagoya 467, Japan.
5. Ph.D., Professor, Department of Hygienic Chemistry, Faculty of Pharmaceutical Sciences. Nagoya City University, Mizuho, Nagoya 467, Japan.
6. Ph.D., Senior Investigator; National Institute for Leprosy Research, Kiyose, Tokyo 204, Japan.
7. D.V.Sc, Room Chief of Laboratory 4, National Institute for Leprosy Research, Kiyose, Tokyo 204, Japan.
8. Ph.D., Research Scientist, Department of Immunology, Otsuka Pharmaceutical Co., Ltd., Tokushima, Tokushima 770, Japan.
Reprint requests to Dr. Onozaki.
Received for publication on 22 June 1993.
Accepted for publication in revised form on 9 September 1993.