- Volume 61 , Number 4
- Page: 563–70
Sensitization potential and reactogenicity of varying doses of BCG plus killed Mycobacterium leprae: an extended study
ABSTRACT
This study is an extension of a previous study on an antileprosy combination vaccine of BCG plus killed Mycobacterium leprae (KML) regarding its sensitization potential and reactogenicity. The study was extended to see if by reducing the dose of BCG in the combination vaccine the incidence of suppurative adenitis could be reduced without a significant reduction in the level of postvaccination skin-test responses. The study included 860 individuals, and three preparations of the combination vaccine [BCG 0.05 mg + 6 x 108 KML (I), BCG 0.05 mg + 5 x 107 KML (II), BCG 0.01 mg + 5 x 107 KML (III)] along with normal saline (IV) were used. Each individual received one of these four preparations by random allocation. They were also tested with Rees' M. leprae soluble antigen (MLSA) and lepromin A 12 weeks after vaccination. Reactions to the MLSA were measured after 48 hr; reactions to lepromin A after 48 hr and 3 weeks. The character and size of the local response at the vaccination site were recorded at the third, eighth, and 15th week postvaccination. The results of the study showed that by halving the dose of BCG in the combination vaccine BCG plus 6 x 108 KML a) the incidence of suppurative regional adenitis was reduced significantly, b) there was no significant change in the postvaccination response at 12 weeks as measured by Rees' MLSA and lepromin A, and c) the evolution of the vaccination lesion was somewhat prolonged. This dose was found satisfactory for use in a comparative antileprosy vaccine trial in South India.RÉSUMÉ
Cette étude est une continuation d'une étude précédente se rapportant à un vaccin anti-lèpre combiné composé de BCG et Mycobacterium leprae tué (MLT) et concernant son pouvoir sensibilisateur et réactogène. L'étude a été prolongée pour voir si en réduisant la dose de BCG dans la combinaison, l'incidence d'adénite suppurée pouvait être réduite sans une réduction significative du niveau des réponses au test cutané postvaccinal. L'étude a concerné 860 personnes, et trois préparations du vaccin combiné [BCG 0.05 mg + 6 x 108 MLT (I), BCG 0.05 mg + 5 x 107 MLT (II), BCG 0.01 mg + 5 x 107 MLT (III)], ainsi qu'une solution saline normale (IV), ont été utilisées. Chaque personne recevait aléatoirement une de ces quatre préparations. Ces personnes ont également été testées par l'antigène soluble de M. leprae de Rees (ASML) et la lépromine A 12 semaines après leur vaccination. Les réactions au ASML ont été mesurées après 48 h; les réactions à la lépromine après 48 h et après trois semaines. Le caractère et le type de réponse locale au site de vaccination ont été enregistrés à la troisième, huitième et quinzième semaines après la vaccination. Les résultats de l'étude ont montré qu'en réduisant de moitié la dose de BCG dans le vaccin combiné BCG plus 6 x 108 MLT : a) l'incidence d'adénites régionales suppuratives était significativement réduite; b) il n'y avait pas de modification significative de la réponse post-vaccinale á 12 semaines comme mesurée par l'ASML de Rees et la lépromine A; c) l'évolution de la lésion vaccinale était quelque peu prolongée. Ce dosage a été jugé satisfaisant pour être utilisé dans un essai comparé de vaccin anti-lèpre dans le Sud de l'Inde.RESUMEN
Este estudio es una extensión de uno anterior sobre una vacuna antileprosa combinada preparada con BCG y Mycobacterium leprae muerto por calor (KML) en relación a su potencial sensibilizante y a su reactogenicidad. El estudio se amplió para investigar si reduciendo la dosis de BCG en la vacuna combinada se reducía la incidencia de adenitis supurativa sin una disminución importante de la reactividad en piel inducida por la vacunación. El estudio incluyó 860 individuos y tres preparaciones de vacunas combinadas [0.05 mg de BCG + 6 x 108 KML (I), 0.05 mg de BCG + 5 x 107 KML (II), 0.01 mg de BCG + 5 x 107 KML (III)], además del uso de solución salina fisiológica (IV). Cada individuo recibió al azar una de estas 4 preparaciones. Doce semanas después de la vacunación, los individuos se probaron con el antígeno soluble de M. leprae de Rees (MLSA) y con lepromina A. Las reacciones al MLSA se midieron a las 48 h y las reacciones a la lepromina a las 48 h y a las 3 semanas. Tres, ocho, y quince semanas después de la vacunación se registró el carácter y el tamaño de la respuesta local en el sitio de aplicación de la vacuna. Los resultados mostraron que reduciendo a la mitad la cantidad de BCG en la vacuna con BCG y 6 x 108 KML: (a) se redujo significativamente la incidencia de adenitis regional supurativa, (b) no hubieron cambios importantes en las respuestas post-vacunales contra el MLSA y la lepromina A, y (c) la evolución de la lesión vacunal fue más prolongada. Esta dosis fue satisfactoria para su uso en un ensayo comparativo de vacunación antileprosa en el Sur de la India.In a study of an antileprosy combination vaccine of BCG plus killed Mycobacterium leprae (KML), three different doses of KML (6 x 108, 5 x 107 and 5 x 106) in combination with 0.1 mg of BCG were used by us before considering this vaccine for a largescale field trial in India (4). The highest dose of KML along with BCG produced maximum sensitization in terms of postvaccination responses to Rees' M. leprae soluble skin test antigen (MLSA) and lepromin A. In comparison, the other two doses of KML in combination with BCG and a fourth group receiving 0.1 mg of BCG alone produced more or less similar, but significantly lower, postvaccination sensitization even though a dose-response effect was discernible. The highest dose of KML in combination with BCG was well tolerated by the vaccinated individuals in terms of local response at the vaccination site. However, 7 persons of the 200 vaccinated with this highest dose developed suppurative regional adenitis as compared to 3, 3 and 0 among those vaccinated with the other three vaccines, respectively. The study was therefore extended to see if by reducing the dose of BCG in the combination vaccine the incidence of suppurative adenitis could be reduced to an acceptable level while still retaining the level of postvaccination sensitization responses. The study was conducted, during 1990, in three villages adjoining the villages wherein the first study was conducted in Tiruthani taluk of Chingleput district, Tamil Nadu, India. The present paper reports on the results of this extended study.
MATERIALS AND METHODS
The following vaccine/control preparations and immunologicals were used in the study:
Vaccine preparations. I. = BCG 0.05 mg + 6 x 108 KML (Lot IV) per dose; II. = BCG 0.05 mg + 5 x 107 KML (Lot IV) per dose; and III. = BCG 0.01 mg + 5 x 107 KML (Lot IV) per dose.
Control preparation. IV. = Normal saline (sodium chloride injection, i.p. 0.9% w/v).
Immunologicals. 1. Rees' M. leprae soluble skin test antigen, 1 µg protein/dose (Lot Wel-4-EFl); 2. Lepromin A, 30-40 million bacilli/ml (Lot J 15-4, 21-7-88).
Freeze-dried BCG vaccine, originally Danish strain, in ampules containing 2 mg of the vaccine, was supplied by the BCG Vaccine Laboratory, Madras, India. Suspensions of vaccine-grade, armadillo-derived KML as well as Rees' MLSA and lepromin A were supplied by the IMMLEP program of the World Health Organization. The KML suspensions were received frozen and were kept in the same condition until their final use. Normal saline was procured locally.
To obtain each of the first two combination vaccines, 2 mg of freeze-dried BCG was reconstituted by adding 4 ml of either of each of the two doses of KML suspension. To obtain the third combination vaccine, 2 mg of freeze-dried BCG was first reconstituted by adding 2 ml of normal saline. After thoroughly shaking the ampule to achieve a uniform suspension, 0.4 ml of this vaccine was added to 4 ml of KML (5 x 108 per ml). Thus the dose of the third combination vaccine was not exact but very close to the dose mentioned. Reconstitution was done just before vaccination. Care was taken to avoid direct sunlight while reconstituting the vaccines, as well as at the time of vaccination. The vaccines were kept cool in ice boxes and were used within 6 hr of the time of reconstitution.
Due to the methods necessary for reconstitution of the vaccines, it was not possible to keep the study completely double blind. However, each of the five "vaccines" were given different codes, and the final preparations were kept in identical-looking, amber-colored ampules. Thus, at the time of actual administration of the vaccines, there were five similar-looking, amber-colored ampules identified with only the code numbers. The key to the codes was available with the statistics section of our Field Unit.
For comparison, the results from our previous study (4) have been presented for the combination vaccine BCG 0.1 mg + 6 x 108 KML (Lot IV) per dose, now designated as I(P).
Study population. Similar to the previous study, it was decided to recruit 200 individuals, 50, 50 and 100 in the age groups 1-6, 7-12 and 13-70 years, respectively, for each study arm with the limited objective of knowing the reactogenicity and sensitizing potential of the combination vaccine BCG plus KML. Leprosy patients and suspects, tuberculosis patients, obviously ill and infirm individuals, and pregnant and lactating women were not included in the study. The distribution of the study subjects by age, separately for the four arms of the trial, is given in Table 1. The control arm was comprised of 260 persons. The opportunity was taken to try one more vaccine based on a cultivable mycobacterium not reported here. Since the quantity of this vaccine was insufficient, only 140 persons could be recruited for this arm of the study and 60 persons who were to receive this vaccine by random allocation were given normal saline. Recruitment into the study was done by individual randomization from each of the three age groups. Out of the 860 individuals recruited, there were 47% males and 53% females. This study area is endemic for leprosy and also is known to have a high level of nonspecific sensitization (7).
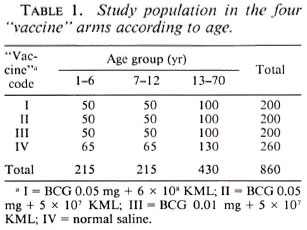
Intake and follow-up procedures. The study subjects received 0.1 ml of one of the vaccine/control preparations, by random allocation, intradermally into the left shoulder at a single site. The size and character of the local response at the vaccination site were recorded at 3, 8 and 15 weeks. The character of the vaccination lesion was recorded as: nodule (N), pustule (P), ulcer (U), pustule with crust (C), dry crust (D), scar partly covered with crust (Q), or completely healed scar (S). Categories D, Q and S represent healed or almost healed lesions.
Twelve weeks after intake, all individuals were tested with 0.1 ml of Rees' MLSA and lepromin A on the upper third dorsum of the left forearm and midvolar part of the right forearm, respectively. The transverse diameter of induration, in millimeters, to Rees' MLSA and to lepromin A were measured at 48 hr. The size of the lepromin A late reaction was measured at the end of 3 weeks. The reader was kept "blind" about the code of the vaccine received by the study subjects. These procedures for intake and follow-up were essentially similar to those followed in the earlier study (4), except that in the earlier study skin-test antigens were given at intake as well as at 12 weeks postvaccination.
Throughout the study period, a medical officer accompanied the field teams. In addition, he visited the villages every week. Individuals reporting any vaccination-related complaints were examined by him and the findings were recorded.
Statistical analysis. The chi-squared test was employed for comparing proportions: the / test (if permissible) or the Mann-Whitney U test was employed to compare two means. For comparing more than two means, the F test (if permissible) or the Kruskal-Wallis test was used.
RESULTS
Local response at the site of vaccination. Information on the character of local responses at the vaccination site for the four vaccine/control preparations used in the study is given in Table 2. As the dose of the vaccines increased, it took a longer time for the vaccination lesions to heal. By week 8, 18%, 39% and 51 % of the lesions had healed (D, Q or S categories) in vaccine groups I, Hand III, respectively (p < 0.01). However, by week 15, the majority of the lesions, 78% in vaccine group I and nearly 90% in vaccine groups II and III, had healed. All of the 352 individuals vaccinated with vaccines I and II, and examined at 15 weeks, and 94% of the 182 individuals vaccinated with vaccine III exhibited a lesion due to vaccination.
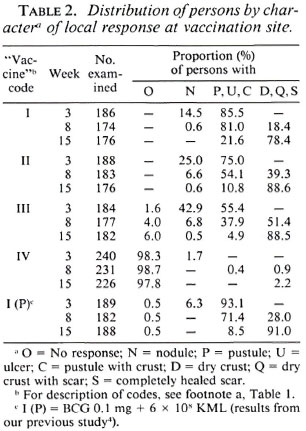
Table 2 also shows the results from the previous study for vaccine I(P). A comparison of the results for vaccines I and I(P) showed that the evolution of the vaccination lesion following vaccine I was slower than that following vaccine I(P). By week 15, 78% of the lesions had healed in group I as compared to 91% in group I(P) (p < 0.01).
Table 3 gives the mean size of the vaccination lesion at weeks 3, 8 and 15 for each of the three vaccine preparations. The mean size of the vaccination lesion increased with age (p < 0.01). It was associated with the dose of the vaccine (p < 0.01), being the highest among persons receiving vaccine I. Considering all of the individuals (i.e., age group 1-70 years), the mean size of the vaccination lesions at week 15 was 10.9, 8.3 and 6.4 mm for vaccines I, II and III, respectively.
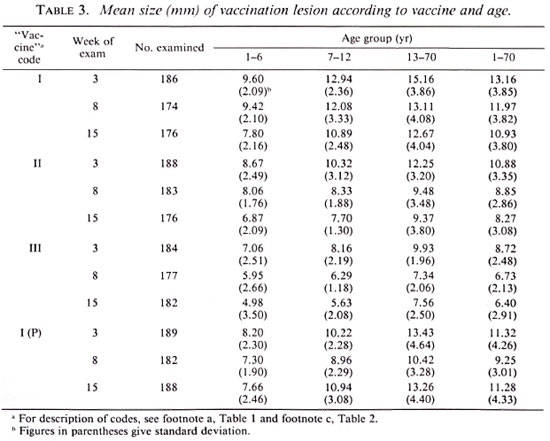
Table 3 also gives the results from the previous study for vaccine I(P). A comparison of the results for vaccines I and I(P) showed that the mean size of the lesion following vaccination with vaccine I was larger (p < 0.01) than that following vaccination with vaccine I(P) at weeks 3 and 8. However, by week 15, when a vast majority of lesions had healed in both of the groups, the mean size of the vaccination lesion was similar in the two groups (p > 0.3).
Postvaccination reactions to Rees' MLSA and lepromin A. Tables 4, 5 and 6 provideinformation on postvaccination skin-test responses measured in terms of Rees'MLSA, early lepromin A and late lepromin A, respectively. The pattern of results observed with respect to the three parameters was very similar. To know the extent of sensitization induced by the vaccine, the mean sizes of postvaccination reactions in individuals receiving vaccines were compared to that in those receiving normal saline. The mean sizes of postvaccination reactions to both Rees' MLSA and lepromin A (early and late) were generally significantly larger (p < 0.05) in all three vaccine groups as compared to those in the normal saline group. Only in three situations-in the age group 13-70 years, lepromin A (early) in vaccine group III, and lepromin A (late) in vaccine groups II and III-were the differences not significant statistically. The mean size of postvaccination reactions was significantly higher (p < 0.01) in individuals receiving vaccine I as compared to those receiving vaccines II or III. However, although a consistent dose-response effect was seen, the difference in mean sizes of post vaccination reactions in individuals receiving vaccines II and III, in most cases, failed to attain statistical significance. The increase in mean postvaccination reactions in the vaccine groups, as compared to that in the normal saline group, was the highest (p < 0.05) in the age group 1-6 years than that observed in age groups 7-12 and 13-70 years, except for lepromin A (late) in vaccine group I.
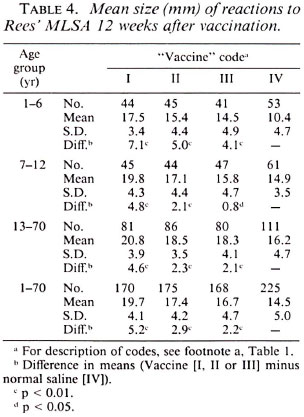
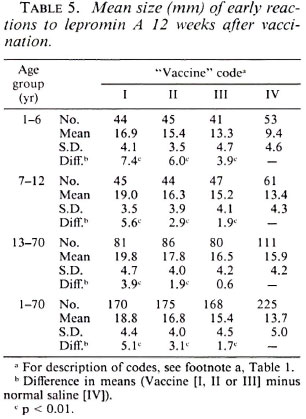
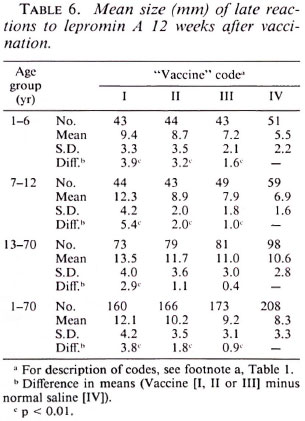
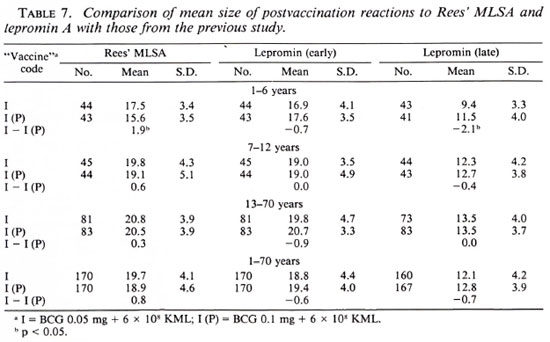
A comparison of postvaccination responses to vaccine I used in the present study and to vaccine I(P) used in the previous study is shown in Table 7. It can be seen that the mean sizes of postvaccination responses to both the antigens in each of the three age groups were similar (p > 0.2) following vaccination with vaccines I and I(P), except in two cases-for Rees' MLSA and late lepromin A in the age group 1-6 years (p < 0.05)-the two differences being in opposite directions.
Complications to vaccination. Only one individual (male, 13 years old) developed suppurative regional adenitis, around week 9, following vaccination with vaccine I. One case each of suppurative adenitis was observed between weeks 16 and 20 following vaccination with vaccines II and III. Healing was prompt after drainage.
A comparison with the results from the previous study showed that the incidence of suppurative regional adenitis was significantly reduced (1 tailed; p < 0.05) following vaccination with vaccine I (1 of 200) as compared to that following vaccination with vaccine I(P) (7 of 200).
DISCUSSION
The objective of the present study was to see if by reducing the dose of BCG in the combination vaccine, the incidence of suppurative adenitis would also be reduced without a significant reduction in the level of postvaccination sensitization. Since an immune response is known to be a function of the logarithm of the dose, reduction of the dose of BCG vaccine by half is unlikely to significantly affect the level of postvaccine sensitization. Edwards, el al. (3) have reported that the effect of halving the number of BCG organisms on the average size of postvaccination tuberculin reactions was hardly detectable, the standard dose of BCG used by them being 0.075 mg/0.1 ml. They also found that the size of the lesions and the frequency of ulcers at the vaccination site decreased as the vaccine was more diluted and that the first dilution (from 1/1 to 1 /10) affected the lesions much more than the allergy. Convit, et al. (1), in their immunoprophylactic study, used  the dose (0.04 mg) of BCG in the combination vaccine for vaccinating individuals who were tuberculin "positive" as compared to the dose (0.2 mg) used in individuals who were tuberculin "negative." In the present study, the dose of BCG was reduced by half along with the same two doses of KML (6 x 108 and 5 x 107) used in the previous study (4). In addition, a third vaccine arm with
the dose (0.04 mg) of BCG in the combination vaccine for vaccinating individuals who were tuberculin "positive" as compared to the dose (0.2 mg) used in individuals who were tuberculin "negative." In the present study, the dose of BCG was reduced by half along with the same two doses of KML (6 x 108 and 5 x 107) used in the previous study (4). In addition, a third vaccine arm with 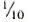 dose of BCG in combination with 5 x 107 KML was selected as the lowest dose that could be considered for immunoprophylaxis.
dose of BCG in combination with 5 x 107 KML was selected as the lowest dose that could be considered for immunoprophylaxis.
By reducing the dose of BCG by half in the combination vaccine, the evolution of the local response at the vaccination site was prolonged. This effect was seen for both the doses of KML (6 x 108 and 5 x 107). Although measurements for local response were not planned beyond week 15 postvaccination, the individuals recruited into the study were kept under surveillance, and it was observed that healing of the vaccination lesions was uneventful in persons in whom it had not healed by 15 weeks. The reasons for this prolongation in the evolution of the vaccination lesion associated with a reduced dose of BCG are not clear.
The results of the study showed that by decreasing the dose of BCG, the incidence of suppurative regional adenitis decreased. In the present study, only one person of the 200 vaccinated with 0.05 mg BCG plus 6 x 108 KML developed suppurative regional adenitis, as compared to 7 persons of the 200 vaccinated with 0.1 mg BCG plus 6 x 108 KML in the previous study.
No skin tests were performed at the time of intake into the study since it was not contemplated to compare the pre-and postvaccination responses to the antigens. The difference in the two responses would be the sum total of effects due to the sensitization by vaccine, boosting of existing sensitization by repeat testing and new sensitization (both specific and nonspecific), and cannot be attributed to vaccination alone. Considering conversion from "negativity" to "positivity" to soluble antigens as a measure of sensitizing capacity of antileprosy vaccines is also problematic and may lead to misleading conclusions due to the effect of regression to mean and other factors (2,4,5) . In view of the above, for measuring the sensitizing effect of the various vaccines in the present study, the mean sizes of postvaccination reactions in the vaccine groups were compared with those in the normal saline group. As explained in our earlier reports (4,5), this procedure helps in obtaining an estimate of the quantum of sensitizing effect that is solely due to the vaccine.
The mean sizes of the postvaccination reactions, to both Rees' MLSA and lepromin, were significantly higher in the vaccine groups as compared to that in the normal saline group, clearly bringing out the sensitizing effect of the vaccines used in the study. This effect was more pronounced among young children. The sensitizing effect was the highest following vaccination with vaccine I. It was not significantly different following vaccination with vaccines II and III, although a consistent dose-response effect was seen. The pattern of postvaccination results was similar for reactions to Rees' MLSA and for Fernandez and Mitsuda reactions to lepromin A. These results are very similar to those observed in the previous study (4).
Postvaccination skin-test results obtained in this study for the vaccine consisting of 0.05 mg BCG plus 6 x 108 KML were compared with those from the previous study (4) for the vaccine consisting of 0.1 mg BCG plus 6 x 108 KML. Although the two vaccines were not used concurrently in the same study, the two studies were undertaken in neighboring villages, using the same batches of the antigens, the same reader and similar procedures. Hence, it is justifiable to compare the results from the two studies. The comparison showed that the mean size of the postvaccination skin-test responses to both Rees' MLSA and lepromin A were similar following vaccination with the two vaccines, i.e., 0.05 mg BCG plus 6 x 108 KML and 0.1 mg BCG plus 6 x 108 KML. In the present study, individuals in the "control" group received only normal saline at intake; in the previous study, they received in addition skin tests with Rees' MLSA and lepromin A. Lepromin by itself is known to induce sensitization (6). In fact, the mean size of the Fernandez reactions to lepromin A 12 weeks after vaccination was 1-2 mm larger in the control group of the previous study as compared to that in the control group of the present study (p < 0.05); they did not differ significantly with respect to reactions to Rees' MLSA and Mitsuda reactions to lepromin A (p > 0.1). It may be noted, hence, that the postvaccination responses to the two vaccines were compared directly without using information from the corresponding "control" groups.
In conclusion, the results of the present study have shown that by halving the dose of BCG in the combination vaccine BCG plus 6 x 108 KML, a) the incidence of suppurative regional adenitis was significantly reduced, b) there was no significant change in the postvaccination response at 12 weeks as measured by Rees' MLSA and lepromin A, and c) the evolution of the vaccination lesion was somewhat prolonged. In view of these results, the combination vaccine, in a dose of 0.05 mg BCG plus 6 x 108 KML, was considered "safe" and "promising" for use in a large-scale comparative vaccine trial in South India.
Acknowledgment. The supply of vaccine-grade M. leprae, Rees' MLSA, and lepromin A from the UNDIV World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR) is gratefully acknowledged. The authors thank the volunteers for their willing participation in the study. They also acknowledge with thanks the hard and meticulous work of the field teams. Thanks are also due to Mr. N. K. S. Brahaspathy for the secretarial assistance.
REFERENCES
1. CONVIT, J., SAMPSON, C, ZUNIGA, M., SMITH, P. G., PLATA, J., SILVA, J., MOLINA, J., PINARDI, M. E., BLOOM, B. R. and SALGADO, A. Immunoprophy lactic trial with combined Mycobacterium leprae BCG vaccine against leprosy: preliminary results. Lancet 339(1992)446-463.
2. DAVIS, C. E. The effect of regression to mean in epidemiological and clinical studies. Am. J. Epidemiol. 104(1976) 493-498.
3. EDWARDS, L. B., PALMER, C. E. and MAGNUS, K. BCG vaccination. Geneva: World Health Organization, 1953, p. 125. WHO Monogr. Ser. 12.
4. GUPTE, M. D., ANANTHARAMAN, D. S., DE BRITTO, R. L. J., VALLISHAYEE, R. S., NAGARAJU, B., KANNAN, S. and SENGUPTA, U. Study of sensitization potential and reactogenicity of BCG with and without various doses of killed Mycobacterium leprae. Int. J. Lepr. 60(1992)340-352.
5. GUPTE, M. D., VALLISHAYEE, R. S., ANANTHARAMAN, D. S. and KANNAN, S. Effect of skin test with M. leprae soluble antigen on reaction to a subsequent test with the same antigen. Int. J. Lepr. 60(1992)54-60.
6. Testing of purified armadillo derived M. leprae in man (Document finalized by the IMMLEP Steering Committee at its meeting, 10-12 June 1981). TDRIMMLEP/SC/Test/81.1:p. 2.
7. Tuberculosis Prevention Trial, Madras. Trial of BCG vaccines in South India for tuberculosis prevention. Indian J. Med. Res. 72 Suppl. (1980)1-74.
1. M.D., D.P.H., Officer-in-Charge; CJIL Field Unit (Indian Council of Medical Research), 271 Nehru Bazaar, Avadi, Madras 600054, India.
2. M.Sc; CJIL Field Unit (Indian Council of Medical Research), 271 Nehru Bazaar, Avadi, Madras 600054, India.
3. M.B.B.S.; CJIL Field Unit (Indian Council of Medical Research), 271 Nehru Bazaar, Avadi, Madras 600054, India.
4. M.A., CJIL Field Unit (Indian Council of Medical Research), 271 Nehru Bazaar, Avadi, Madras 600054, India.
Received for publication on 21 May 1993.
Accepted for publication in revised form on 29 September 1993.