- Volume 61 , Number 4
- Page: 571–80
Serological response in leprosy and tuberculosis patients to the 18-kDa antigen of Mycobacterium leprae and antigen 85B of Mycobacterium bovis BCG
ABSTRACT
Two enzyme-linked immunosorbent assays (ELISAs) for the detection of leprosy antibodies were developed. These assays were based on the recombinant 18-kDa protein of Mycobacterium leprae and the 85B antigen of M. bovis. Sera f rom leprosy and tuberculosis patients were analyzed, and a phenolic glycolipid-I (PGL-I) ELISA was used as a reference test. The 18-kDa ELISA reached a sensitivity of 70% on lepromatous leprosy sera. The corresponding results for the 85B and the PGL-I ELISAs were 93% and 96%, respectively. The sensitivity on tuberculoid leprosy sera was 72% for the 18kDa antigen, 95% for the 85B antigen, and 60% for the PGL-I antigen. The 18-kDa antigen ELISA was found to have crossreactivity to sera f rom patients with tuberculosis, while the 85B and PGL-I assays had high specificity.RÉSUMÉ
On a développé deux tests immuno-enzymatiques (ELISA) pour la détection des anticorps vis-à-vis de la lèpre. Ces tests sont basés sur la protéine de 18 kDa de Mycobacterium leprae et l'antigène 85B de M. bovis. Des serums de patients lépreux et tuberculeux ont été analysés, et un ELISA basé sur le glycolipide phénolique-I (GLP-I) a été utilisé comme test de référence. L'ELISA de 18-kDa a atteint une sensibilité de 70% sur des serums lépromateux. Les résultats correspondants pour les ELISA 85B et GLP-I étaient respectivement de 93% et 96%. La sensibilité vis-à-vis de serums de lèpre tuberculoide étiat de 72% pour l'antigène de 18-kDa, 95% pour l'antigène 85B, et 60% pour l'antigène GLP-I. On a trouvé pour l'ELISA basé sur l'antigène 18-kDa une réactivité croisée vis-à-vis des serums de patients tuberculeux, alors que les ELISA 85B et GLP-I avaient une spécificité élevée.RESUMEN
Se desarrollaron dos inmunoensayos enzimáticos (ELISAs) para la detección de anticuerpos anti-Mycobacterium leprae en el suero de pacientes con lepra y en el suero de pacientes con tuberculosis. En estos ensayos se utilizaron como antígenos la proteína recombinante de 18 kDa del M. leprae y el antígeno 85B del M. bovis. Como referencia se utilizó un ELISA utilizando glicolípido fenólico-I (PGL-I). Las sensibilidades de los ELISAs con los sueros de los pacientes lepromatosos fueron del 70% con la proteina de 18 kDa, del 93% con el antígeno 85B, y del 96% con el PGL-I. En el caso de los sueros de los pacientes con lepra tubereuloide, las sensibilidades fueron del 72% para el antígeno de 18 kDa, del 95% para el antígeno 85B, y del 60% para el PGL-I. El antígeno de 18 kDa también fue reconocido por los sueros de los pacientes con tuberculosis pero los antígenos 85B y PGL-I fueron altamente específicos para la lepra.Major protein and glycoprotein antigens of Mycobacterium bovis/M. tuberculosis have crossreactive counterparts in M. leprae (8). Consequently, it has been difficult to identify mycobacterial protein antigens giving rise to a disease-specific antibody response. It has recently been demonstrated that sera from patients with lepromatous leprosy react preferentially with certain protein antigens of M. bovis, M. tuberculosis and M. leprae (29). A crude M. leprae sonicate used for antibody detection in enzymelinked antibody assays (ELISAs) has not attained the required specificity for a diagnostic test. The monoclonal antibody (mAb) technology initiated a search for species-specific epitopes rather than species-specific proteins, but it was not until the discovery of the glycolipids that specific assays could be developed. Immunochemical studies showed that mycobacterial species contain glycolipid antigens at the cell surface (4). A glycolipid antigen called phenolic glycolipid-I (PGL-I) was purified from M. leprae (14). The IgM response to this antigen was found to have a high specificity and high sensitivity for the diagnosis of lepromatous leprosy (0.96) but a lower sensitivity for the diagnosis of tuberculoid leprosy (0.62) (4). The terminal sugars of PGL-I were recognized as the immunodominant species-specific determinants, and have been synthesized and attached to bovine serum albumin to achieve immunological activity in antibody assays (6,7). A glucoconjugate was developed for the PGL ELISA (6). The sensitivity and specificity was similar to those achieved with the native glycolipid. The PGL-I ELISA is considered the best choice for comparison with new assays.
A number of genes of M. leprae have been cloned, and a number of recombinant proteins picked out using mABs (31). The 18kDa protein is of special interest because mABs raised against it crossreact with very few other mycobacterial pathogens (26). The 18-kDa antigen has been expressed in both Escherichia coli and a yeast vector (2,3). This has made pure 18-kDa antigen available in large quantities for evaluation of its diagnostic potential in antibody assays.
There is a well-documented similarity in antigen composition between M, leprae and M. tuberculosis/M. bovis (13). This implies that the immune response to BCG proteins may be relevant for leprosy. The BCG 30-to-32-kDa protein doublet corresponds to the antigen 85 complex in the crossed immunoelectrophoresis reference system for BCG (8) and M. tuberculosis (27). This complex consists of three closely related components, termed 85A, 85B and 85C, which are encoded by individual genes (30). They are actively secreted by M. tuberculosis and M. bovis during culture (28) and probably by M. leprae as well (18). Recent studies have demonstrated that leprosy and tuberculosis patients have antibodies to these crossreactive immunodominant proteins (19). The 85B antigen was found to provide the best distinction between lepromatous and tuberculoid leprosy patients, and the response among patients with active tuberculosis was higher than for those with inactive forms of the disease (19).
The aim of the present study was to evaluate the diagnostic potential of recombinant 18-kDa antigen of M. leprae, I3CG antigen 85B and PGL-I in ELISA performed on sera from patients with leprosy or tuberculosis.
MATERIALS AND METHODS
Sera
Sera were collected from 47 leprosy patients (20 from Nepal and 27 from Ethiopia) classified according to the Ridley-Jopling scale (22). The classifications of the leprosy patients were performed at the Armauer Hansen Research Institute in Ethiopia and in the Anandaban Leprosy Hospital in Nepal as lepromatous leprosy (LL) = 27, borderline tuberculoid leprosy (BT) = 20, and 10 household contacts (HC) to leprosy patients (from Nepal). Sera were also collected from 28 Swedish patients with tuberculosis (TB; in ages from 5 to 94 years, 21-culture positive; in the culture-negative group were 2 children each with a typical picture of pulmonary tuberculosis and a culture-positive mother and 5 adults with a strong clinical suspicion of tuberculosis plus strong positive PPD or close TB contacts) and 50 normal controls (NC; from Sweden, 20 sera from adult blood donors and 30 sera from children).
Antigens
18 kDa. A recombinant plasmid (pML3) with an 18-kDa gene was used to transform the E. coli strain DH5 to provide expression of the M. leprae 18-kDa protein. After cultivation of the E. coli, lysis was performed by using 8 M urea. The 18-kDa protein was purified to homogeneity by precipitation and high pressure liquid chromatography (2).
85B. The BCG antigen 85B was purified from M. bovis BCG Copenhagen culture fluid as described elsewhere (14). The purity of the antigen was controlled by using sensitive crossed immunoelectrophoresis. Only one line corresponding to the 85B antigen was observed by using antibodies to the 85 complex in the top gel.
PGL-I. The terminal sugar of PGL-I, as a synthetic trisaccharide covalently coupled to bovine serum albumin (BSA), was kindly provided by Dr. P. J. Brennan (Colorado State University, Fort Collins, Colorado, U.S.A.).
ELISA
Nunc Immunolone plates were used in all ELISAs (Nunc, Copenhagen, Denmark).
PGL-I ELISA. For detection of IgM antibodies against the PGL-I antigen, the plates were coated with 10 µg/well of the synthetic trisaccharide of the PGL-I coupled to BSA. Coating was performed at 37ºC overnight. After washing three times with phosphatebuffered saline (PBS) containing 0.1 % Tween 20 (PBS-T), 100 µl of 10% normal goat serum was added for blocking, and the plates were incubated for 1 hr at room temperature. After subsequent washing, 50 µl test serum was added at a dilution of 1/500 followed by incubation at 37ºC for 1 hr. After washing three times with PBS-T 50 µl peroxidase-labeled goat antihuman IgM was added at a dilution of 1/1000 (Dakopatt). After incubation for 1 hr at 37ºC and washing five times, the substrate ortho-phenyldiamine (OPD) was added (50 µl, 0.66 mg/ ml), and the reaction was stopped by adding 2.5 N H2SO4 after 17 min. Optical density (OD) was measured using an ELISA reader with a 492 nm filter.
18-kDa ELISA. The ELISA plates were coated with the 18-kDa antigen in bicarbonate buffer (pH = 9.6) at a concentration of 1 µg/well. After incubation overnight at 4ºC the plates were washed three times with PBS-T. The plates were then blocked by 1% BSA at 37ºC for 1 hr. After washing three times, test sera were added at a dilution of 1/200 in PBS-T, followed by another incubation for 1 hr at 37ºC, and then washed three times again. The secondary antibody, biotinylated anti-human IgG (Sigma) diluted 1/500 in PBS-T, was absorbed for 1 hr at room temperature, and the plates were washed three times as before. Streptavidine biotin horseradish peroxidase conjugate (Sigma), diluted 1 /1000 in PBS, was allowed to react for 1 hr at 37ºC, and the wells were washed five times with PBS. The color was developed by using OPD. The reaction was stopped after 4 min by adding 2.5 N H2SO4 and the absorbance was read in an automated ELISA reader at 492 nm.
BCG 85B ELISA. The BCG 85B antigen was dissolved in carbonate buffer, pH 9.6, applied to the plate at a concentration of 0.5 µg/well, and incubated for 48 hr at 4ºC in a moist chamber. The plate was then washed three times with PBS-T and blocked by 0.05% BSA in PBS-T at 37ºC for 30 min. After washing three times, 100 µl test serum diluted 1/50 in PBS-T was added, and the plate was incubated for 30 min at 37ºC. Washing was repeated and 100 µ/well of peroxidase-conjugated goat antihuman IgG at a dilution of 1/1000 was applied. Incubation in 37ºC for 30 min was followed by washing five times and 50 µl/well of 0.66 mg/ml OPD was allowed to react for 4 min. The reaction was stopped by adding 50 µl/weel 2.5 NH2SO4 , and the absorbance was read in an automated ELISA reader at 492 nm.
Sera from all groups of patients/controls were, for each antigen, randomly placed on the ELISA plates.
The cut-off value for each of the three ELISAs was calculated from the absorbance values from the healthy controls by adding 2 standard deviations to the mean value (X + 2 x S.D.).
Statistical analysis
Differences in mean optical density (OD) in the various groups were measured by the Student's t test. Differences in the proportion of seropositives were tested by the chisquared test. Differences in OD values in different groups were analyzed with Wilcoxon's signed rank test.
RESULTS
The OD values for the different sera as analyzed with the three different assays are demonstrated in Figure 1. The OD values for LL sera had a wide range from low to high levels in the PGL-I ELISA and the 85B ELISA, while the OD values for the 18-kDa ELISA were scattered around the cut-off level with only a few sera at high OD levels. For BT sera, the OD values for the PGL-I assay were close to the cut-off point except for two sera with high absorbance. The OD values of the healthy controls are also shown in Figure 1.
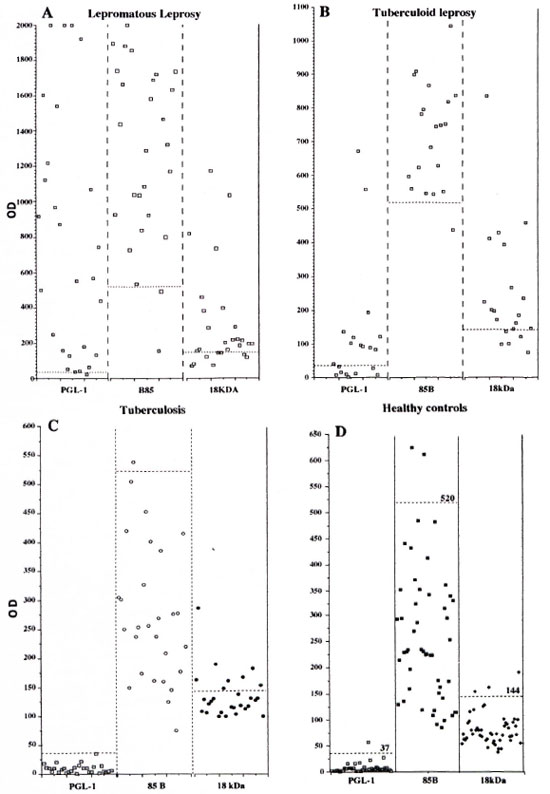
Fig. 1. Optical density results for the four different serum groups, lepromatous leprosy (A), tuberculoid leprosy (B), tuberculosis (C) and healthy controls (D) analyzed with three different ELISAs based on the antigens PGL-I, 18-kDa leprosy protein, 85B M. bovis protein, (---) Cut-off values for each assay.
A comparison of the results from the three different serum groups are presented in Figure 2 as the mean OD values with one standard deviation. There are significant differences between the OD results from the 18-kDa assay for healthy donors and BT (p < 0.001) and LL (p < 0.001) patients. This was also the case in the 85B assay for BT patients (p < 0.001) and LL patients (p < 0.001). As seen in Figure 2 there is also a marked difference in the mean OD for lepromatous leprosy and tuberculoid leprosy sera in the PGL-I ELISA. To a lesser extent this was also the case for the 85B and 18-kDa assays. The PGL-I ELISA and 85B ELISA gave mean ODs on the same levels as TB and BT sera, while there was a difference between these serum groups in the 18-kDa ELISA.
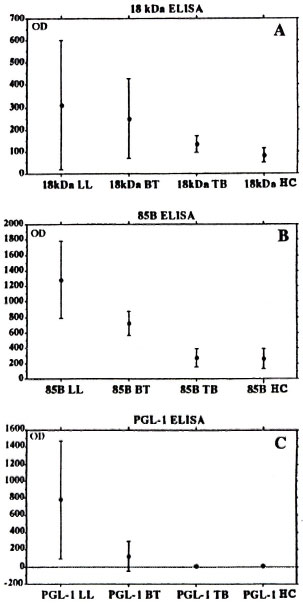
Fig. 2. One standard deviation error bars of OD means for A) 18-kDa ELISA, B) 85B ELISA and C) PGL-I ELISA on sera from patients with lepromatous leprosy (LL), tuberculoid leprosy (BT), tuberculosis (TB) or from healthy controls (HC).
The relation of the number of seropositives in the three assays used on the different leprosy groups is seen in Figure 3. As seen in this figure, from a group of lepromatous leprosy sera 26 of 27 (96%) were positive for two antigens, and of these, 17 of 27 sera (63%) were positive for all three antigens. The corresponding figures for sera from tuberculoid leprosy patients are 18 of 20 (95%) and 9 of 20 (45%), respectively. For LL sera, the PGL-I and 85B ELISAs picked up more positives than did the 18-kDa ELISA. Among the 20 BT sera 19 were positive in the 85B ELISA and only 1 of them in the 85B ELISA alone, while 15 of 20 also were positive in the 18-kDa ELISA and 12 of 20 were also positive in the PGL-I ELISA.
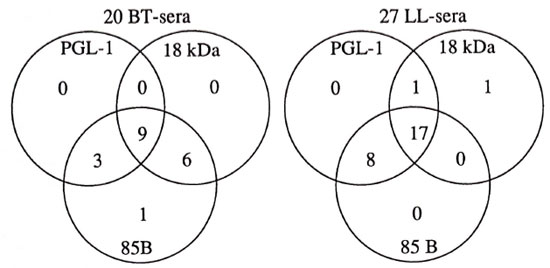
Fig. 3. Relation of positive ELISA results in the three different assays for sera from patients with tuberculoid leprosy (BT) and lepromatous leprosy (LL).
For TB patients, the relation of the sera negative in the three assays is presented in Figure 4. Eight TB sera were positive in the 18-kDa ELISA, while 19 of 28 were negative in all three assays and 27 of 28 were negative in both the PGL-I ELISA and the 85B ELISA.
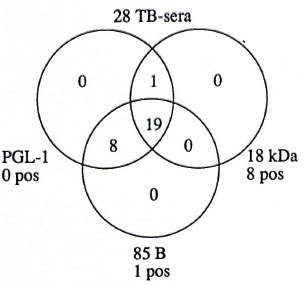
Fig. 4. Relation of negative results in the three assays based on the antigens PGL-I, 18-kDa leprosy protein and 85B M. bovis protein on sera from patients with tuberculosis is shown within the circles; number of sera with positive results are shown outside circles.
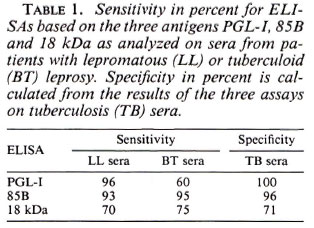
The sensitivity and specificity for each assay are presented in Table 1. Results from the three different ELISAs on TB sera have been used for the calculation of the specificity since tuberculosis is a risk group for serological crossreactions. One of the 10 family contacts of leprosy patients had positive serology in the PGL-I ELISA. The serum had a high OD value and was also positive in the two other assays. The 18-kDa ELISA was positive in 7 of 10 and the 85B ELISA in 7 of 10. Five of these positives were positive in both the 18-kDa and the 85B ELISA.
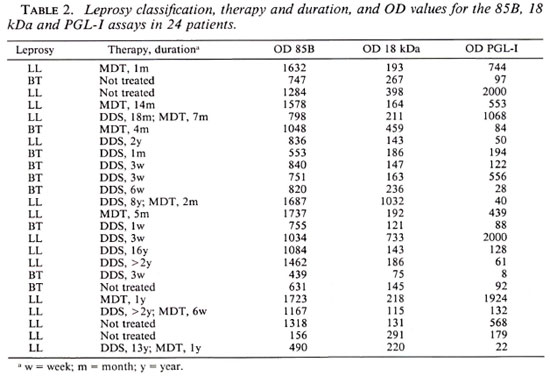
The treatment status was uncertain for a majority of the leprosy patients. However, in 24 patients we had valid information on this point (Table 2). Ten patients (9 LL and 1 BT) had been treated for more than 6 months and 14 patients (6 LL and 8 BT) for less than 6 months. The OD values from the three assays were compared for the two treatment groups and no statistically significant differences were found (Wilcoxon's signed-rank test). The OD values from the three assays for the LL patients were compared for the two treatment groups and a statistically significant difference was found for the PGL-I assay (p = 0.028) but not in the two other assays.
DISCUSSION
The 18-kDa antigen was chosen for this evaluation due to reports that the murine mAb L-5 which recognized the 18-kDa protein was specific for M. leprae. If immunogenic in leprosy patients, the 18-kDa protein would have potential as an antigen in a diagnostic test for M. leprae antibodies. No clinical evaluation of this antigen had yet been reported when the analyses in the present study were performed. A recent report, however, by Roche, et al. (") describes the antibody response to 18 kDa in sera from patients with leprosy or tuberculosis. They describe 44% seropositives among multibacillary (MB) patients, but very few among paucibacillary (PB) patients. However, 47% of the patients with tuberculosis had an antibody response to the 18-kDa antigen. In the present study the corresponding figures are 70% for MB patients, 75% for PB patients, and 29% for patients with tuberculosis. The explanation for these differences is not obvious, but might be due to the different classification of PB patients, different control groups, and calculation of the cut-off value. If this value, in the present study, is set at three S.D. over mean OD for the control group, the figures would change to 57% positives for lepromatous leprosy, 39% for tuberculoid leprosy and 10% for tuberculosis.
Different frequencies of tuberculosis or BCG vaccination in the studied leprosy groups also may have influenced the results. In this study we found, as did Roche, et al., an unexpectedly high crossreactivity to 18 kDa by antibodies in sera from TB patients. Previous studies had reported the 18-kDa protein to be specific for M. leprae (5). Recent data, however, indicate that the 18kDa protein may share epitopes with other mycobacterial species (10). Polyclonal antisera raised against recombinant M. leprae 18-kDa protein showed crossreactivity with sonicated M. tuberculosis. A T-cell proliferative response to the 18-kDa protein also has been observed in 70% of BCG-vaccinated British blood donors (9).
In vivo experiments have indicated that mycobacterial proteins secreted during culture may be of importance in the immune response to mycobacterial infection. Since cultures of M. leprae could not be studied in vitro, the well-known similarity in antigen composition to M. bovis BCG has been demonstrated to be relevant in the study of the immune response to M, leprae (19). These antigens, with molecular masses of 28 kDa and 30-33 kDa, also are detected from sonicated extracts of slow-growing M. marinum, M. kansasi and M. tuberculosis, suggesting a broad distribution of these antigens within the genus. Pessolani, et al. (20) reported in 1989 a serologic response to these antigens in sera from patients with lepromatous leprosy but not from patients with tuberculoid leprosy or tuberculosis, nor in sera from healthy persons previously BCG vaccinated. The 28-30-kDa doublet protein has been identified as components of the BCG 85 complex (8,28,29) , also known as fibronectin-binding proteins (1). In a 1991 study Pessolani, et al. (19) suggested that the secreted protein with a molecular mass of 28 kDa might be the corresponding B-component of the BCG 85 complex. The M. leprae homolog of the 85B antigen has recently been cloned (15) and has also been demonstrated in the tissue of leprosy patients (12).
The A component of the BCG 85 complex has been identified as a 32-kDa protein corresponding to the P30 component in the 28-to-30-kDa doublet. This antigen has been shown to be immunodominant when antibodies were evaluated in active pulmonary tuberculosis (24). In a recent comparison (19) using an ELISA, significantly higher antibody levels were found to 85B than to 85A in sera from patients with lepromatous leprosy. Only low antibody titers were found in sera from patients with tuberculoid leprosy or tuberculosis. In the present study the percent positives were 93% for lepromatous leprosy, 95% for tuberculoid leprosy and 4% for tuberculosis.
Since other mycobacterial infections are theoretically the main risk groups for crossreactions in a leprosy diagnostic test, the low reactivity in the TB sera is surprising. To some extent this could be explained by high age and low disease activity in these patients except that young patients with more active tuberculosis also had low reactivity. This is in contrast to previous studies where high numbers of patients with tuberculosis had antibodies to the 85 complex (17,25). As for the 18-kDa assay, we noted a high number of positives for tuberculoid leprosy patients. This could be due to some misclassification of PB patients. Speaking against this explanation are the PGL-I ELISA results of this group which, in frequency of seropositives, are on the same level as has been reported from other studies (4). In PB leprosy patients the clinical diagnosis is more difficult than in MB patients, and previous studies have demonstrated that the greatest diagnostic potential for a serologic assay lies in the diagnosis of PB leprosy, although the sensitivity of the assay is much higher in the MB disease (17). With an ELISA-inhibition test based on the competition between serum antibodies and a monoclonal antibody against the 36-kDa leprosy protein, Klatser, et al. (16) reported a sensitivity of 91% for PB patients. These results, as with the 85B ELISA results from the present study, are promising but need further investigation on a larger group of clinically well-defined PB patients.
In the present study a significant difference in antibody levels to PGL-I was observed between long-treated and short- or not treated lepromatous patients. A corresponding difference could not be observed for the 85B and 18-kDa antigens. Drowart, et al. (11) recently reported decreasing levels of antibodies against the 85B and the PGL-I antigens during treatment, but in several cases high antibody levels were still seen after more than 2 years.
In the present study the 18-kDa ELISA did not give sufficient sensitivity or specificity fora diagnostic assay. The 85B ELISA was found to be comparable with the PGL-I ELISA on sera from lepromatous leprosy patients but more sensitive on sera from patients with borderline tuberculoid leprosy.
Acknowledgment. This study was supported by grants from the Swedish Leprosy Mission, Ôrebro, Sweden; Ôrebro county council research unit and Folke Norbring's research foundation; Department of Infectious Diseases, Lund, Sweden. Sera for this study were kindly provided by Dr. W. J. Britton, Clinical Immunology Research Center, University of Sydney, Sydney, Australia, and Ronny Ohman, Department of Bacteriology, University of Gothenburg, Sweden. For excellent technical assistance we want to thank Mrs. Ulla Larson.
REFERENCES
1. ABOUD-ZEID, C, RATTLIFF, T. L., WIKER, H. G., HARBOE, M., BENNEDSEN, J. and ROOK, G. Characterization of fibronectin-binding antigen released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect. Immun. 56(1988)3046-3051.
2. BOOTH, R. J., GRANDISON, P. M., PRESTIDGE, R. L. and WATSON, J. D. The use of a universal yeast expression vector to produce an antigenic protein of Mycobacterium leprae. Immunol. Lett. 19(1988)65-69.
3. BOOTH, R. J., HARRIS, D. P., LOVE, J. M. and WATSON, J. D. Antigenic proteins of Mycobacterium leprae: complete sequence of the gene for the 18-kDa protein. J. Immunol. 140(1988)597-603.
4. BRENNAN, P. J. Structure of typing antigens of atypical mycobacteria; a brief review of present knowledge. Rev. Infect. Dis. 3(1981)903-913.
5. BRITTON, W. J., HELLQVIST, L., BASTEN, A. and RAISON, R. L. Mycobacterium leprae antigens involved in human immune responses; identification of four antigens by monoclonal antibodies. J. Immunol. 135(1985)4171-4177.
6. CHO, S.-N., FUJIWARA, T., HUNTER, S. W., REA, T. H., GELBER, R. H. and BRENNAN, P. J. Use of an artificial antigen containing the 3,6-di-O-metyl-β-D-glucopyranosol epitope and for the serodiagnosis of leprosy. J. Infect. Dis. 150(1984)311-322.
7. CHO, S.-N., YANAGIHARA, D. L., HUNTER, S. W., GELBER, R. H. and BRENNAN, P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
8. CLOSS, O., HARBOE, M., AXELSEN, N. H., BUNCH-CHRISTENSEN, K. and MAGNUSSON, M. The antigens of Mycobacterium bovis strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand. J. Immunol. 12(1980)249-263.
9. DOCKRELL, H. M., STOKER, N. G., LEE, S. P., JACKSON, M., GRANT, K. A., JOUY, N. F., LUCAS, S. B., HASAN, R., HUSSAIN, R. and MCADAM, K. P. W. J. T-cell recognition of the 18 kilodalton antigen of Mycobacterium leprae. Infect. Immun. 57(1989)1979-1983.
10. DOHERTY, T. M . Epitopes recognized by the murine immune response to the IS kilodalton protein of Mycobacterium leprae, Ph.D. thesis, University of Auckland, 1991.
11. DROWART. A., CHANTEAU, S., HUYGEN, K., DE COOK, M., CARTEL, J.-L., DE BRUYN, J., LAUNOIS, P., YERNAULT, J.-C. and VAN VOOREN, J.-P. Effects of chemotherapy on antibody levels directed against PGL-I and 85A and 85B protein antigens in lepromatous patients. Int. J. Lepr. 61(1993)29-34.
12. ESPITA, C, CERVERA, I., GONZALES, R. and MANCILLA, R. A 38 kD Mycobacterium tuberculosis antigen associated with infection; its isolation and serological evaluation. Clin. Exp. Immunol. 77(1989)373-377.
13. HARBOE, M., CLOSS, O., BJORVATN, B., KRONVALL, G. and AXELSEN, N. H. Antibody response in immunisation with Mycobacterium leprae. Infect. Immun. 18(1977)792-805.
14. HUNTER, S. W., FUJIWARA, T. and BRENNAN, P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J. Biol. Chem. 257(1982)15072-15078.
15. HUYGEN, K., VAN VOOREN, J. P., TURNEER, M., BOSMANS, M., DIERCKX, P. and DE BRUYN, J. Specific lymphoprolifcration, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand. J. Immunol. 27(1988)187-194.
16. KLATSER, P. R., DEWIT, M. Y. L. and KOLK, H. J. An ELISA-inhibition test using monoclonal antibody for serology of leprosy. Clin. Exp. Immunol. 62(1985)468-473.
17. LEFFORD, M. J., HUNEGNAW, M. and SIWIK, E. The value of IgM antibodies to PGL-I in the diagnosis of leprosy. Int. J. Lepr. 59(1991)432-440.
18. PESSOLANI, M. C. V. and BRENNAN, P. J. Mycobacterium leprae produces extracellular homologs of the antigen 85 complex. Infect. Immun. 60(1992)4452-4459.
19. PESSOLANI, M. C, PERALTA, J. M., RUMJANEK, F. D., GOMES, H. M., DE MELO MARQUES, M. A., ALMEIDA, E. E. C, SAAD, M. H. F. and SARNO, E. N. Serology and leprosy: immunoassays comparing immunoglobulin G antibody responses to 28- and 30-kilodalton proteins purified from Mycobacterium bovis BCG. J. Clin. Microbiol. 29(1991)2285-2290.
20. PESSOLANI, M. C, RUMJANEK, F. D., GOMES, H. M., DE MELO MARQUES M. A., DE MELO, F. S. F. and SARNO, E. N. Serological response of patients with leprosy to a 28- to 30-kilodalton protein doublet from early cultures of Mycobacterium bovis. J. Clin. Microbiol. 27(1989)2184-2189.
21. RAMBUKKANA, A., DAS, P. K., BURGRAAF, J. D., YONG, S., FARBER, W. R., THOLE, J. E. and HARBOE, M. Heterogeneity of monoclonal antibody-reactive epitopes on mycobacterial 30-kilodalton-region proteins and secreted antigen 85 complex and demonstration of antigen 85B on the Mycobacterium leprae cell surface. Infect. Immun. 60(1992)5172-5181.
22. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
23. ROCHE, P. W., PRESTIDGE, R. L., WATSON, J. D. and BRITTON, W. J. Antibody responses to the 18-kDa protein of Mycobacterium leprae in leprosy and tuberculosis patients. Int. J. Lepr. 60(1992)201-206.
24. STROEBEL, A., DANIEL, T. M., LAU, J. H. K., LEONG, J. C. Y. and RICHARDSON, H. Serologic diagnosis of bone and joint tuberculosis by an enzyme-linked immunosorbent assay. J. Infect. Dis. 146(1982)280-283.
25. THOLE, J. E., SCHONINGH, R., JANSSON, A. A., GARBE, T., CORNELISSE, Y. E., CLARK-CURTIS, J. E., KOLK, A. H., OTTENHOFF, T. H., DE VRIES, R. R. and ABOUD-ZEID, C. Molecular and immunological analysis of a fibronectin-binding protein antigen secreted by Mycobacterium leprae. Mol. Microbiol. 6(1992)153-163.
26. WATSON, J. D. Leprosy: understanding protective immunity. Immunol. Today 10(1989)218-221.
27. WIKER, H. G., HARBOE, M., BENNEDSEN, J. and CLOSS, O. The antigens of Mycobacterium tuberculosis, H37Rv, studied by crossed immunoelectrophoresis; comparison with a reference system for Mycobacterium bovis, BCG. Scand. J. Immunol. 27(1988)223-239.
28. WIKER, H. G., HARBOE, M. and LEA, T. Purification and characterization of two protein antigens from the heterogenous BCG 85 complex in Mycobacterium bovis BCG. Int. Arch. Allergy Appl. Immunol. 81(1986)289-306.
29. WIKER, H. G., HARBOE, M., NAGAI, S., PATARROYO, M. E., RAMIREZ, C. and CRUZ, N. MPB59, a widely cross-reacting protein of Mycobacterium bovis BCG. Int. Arch. Allergy Appl. Immun. 81(1986)307-314
30. WIKER, H. G., SLETTEN, K., NAGAI, S. and HARHOE. M. Evidence for three separate genes encoding the proteins of the mycobacterial antigen 85 com plex. Infect. Immun. 58(1990)272-274.
31. YOUNG, R. A, MEHRA, V., SWEETSER, D., BUCHANAN, T. M., CLARK-CURTISS, J., DAVIS, R. W. and BLOOM, B. R. Genes for the major protein antigens of Mycobacterium leprae. Nature 316(1984)451-452.
1. M.D., Ph.D., Department of Infectious Diseases; Department of Clinical Microbiology and Immunology, Orebro Medical Center Hospital, 70185 Örebro, Sweden.
2. M.D., Ph.D., Assistant Professor, Department of Clinical Microbiology and Immunology, Orebro Medical Center Hospital, 70185 Örebro, Sweden.
3. M.D., Ph.D., Institute of Immunology and Rheumatology, University of Oslo, Oslo, Norway.
4. Ph.D., Professor, Department of Molecular Medicine, University of Auckland, Auckland, New Zealand.
Received for publication on 30 March 1993.
Accepted for publication in revised form on 18 August 1993.