- Volume 60 , Number 2
- Page: 189–94
Preliminary study of cellular immunity to Mycobacterium leprae protein in contacts and leprosy patients
ABSTRACT
Because of the good results obtained in the mononuclear cell (T lymphocyte) proliferative response in tuberculoid leprosy patients and family contacts and healthy Mitsuda-positive volunteers using Mycobacterium leprae soluble extract, we prepared different protein fractions from the soluble extract. We used the T-cell Western blot technique with separation by electrophoresis in SDS-polyacrylamide gels and transfer onto nitrocellulose membranes. Each unstained blot was converted into 18 fractions of antigen-bearing particles and tested with peripheral blood mononuclear cells from 21 individuals including Mitsuda-positive contacts, vaccinated lepromatous leprosy (LL) patients, borderline tuberculoid (BT) patients, and unvaccinated lepromatous patients. The stimulation index (SI) of the contacts was higher to the different fractions in comparison with the leprosy patients. They showed four peaks of stimulation to fractions 66-55, 45-29, 22-18, and 14 kDa. The second highest responders were BT patients, followed by vaccinated LL patients. The unvaccinated patients did not respond significantly to any of the fractions (SI <' 1).RÉSUMÉ
A cause des bons résultats obtenus dans la réponse proliferative des cellules mononucléaires (lymphocytes T) à un extrait soluble de Mycobacterium leprae chez des patients présentant une lèpre tuberculoide et des contacts familiaux et des volontaires en bonne santé positifs au test de Mitsuda, nous avons préparé différentes fractions protéiques à partir de l'extrait soluble. Nous avons utilisé la technique de Western blot des cellules T avec séparation par électrophorèse en gel de SDS-polyacrylamide et transfert sur membrane de nitrocellulose. Chaque "blot" non teinté fut converti en 18 fractions de particules porteuses d'antigènes et testé avec des cellules mononucléaires du sang périphérique de 21 personnes y compris des contacts positifs pour le Mitsuda, des patients lépromateux (LL) vaccinés, des patients borderline tuberculides (BT), et des lépromateux non vaccinés. L'index de stimulation des contacts (IS) était plus élevé pour les différentes fractions par rapport aux malades de la lèpre. Ils ont montré quatres pics de stimulation pour les fractions 66-55, 45-29, 22-18 et 14-kDa. Le deuxième groupe à présenter la réponse la plus forte était les patients BT, suivis par les patients LL vaccinés. Les patients non vaccinés n'ont répondu de manière significative à aucune des fractions (IS < 1).RESUMEN
Las células mononucleares (linfocitos T) de pacientes con lepra tubereuloide, de sus contactos sanos, y de voluntarios sanos no relacionados, proliferan en respuesta al estímulo con un extracto soluble del Mycobacterium leprae. En este estudio se analizó el efecto de diferentes fracciones proteicas del extracto soluble del M. leprae sobre la respuesta proliferativa de las células. El extracto soluble se fraccionó por clectroforésis en poliacrilamida-SDS, las fracciones se transfirieron a membranas de nitrocelulosa, y de cada membrana se prepararon 18 suspensiones de partículas de NC con diferentes fracciones antigénicas. Las partículas se probaron con las células mononueleares de la sangre de 21 individuos (contactos Mitsuda-positivos, pacientes lepromatosos vacunados y no vacunados, y pacientes con lepra tuberculoide subpolar). En general, el índice de estimulación (IE) de los contactos fue mayor que el de los pacientes con lepra. Los contactos mostraron 4 picos de respuesta con las fracciones de 66-55,45-29, 22-18, y 14 kDa. Los segundos mejores respondedores fueron los pacientes con lepra tuberculoide subpolar, seguidos por los pacientes lepromatosos vacunados. Los pacientes lepromatosos no vacunados no respondieron significativamente a ninguna de las fracciones (IE < 1).The peripheral blood mononuclear cell (PBMC) responses in leprosy show broad variation according to the disease spectrum. There is a good response at the tuberculoid pole characterized by an active response in cell-mediated immunity (CMI). In contrast, cells of patients from the lepromatous pole do not proliferate in the presence of specific antigen.
Mycobacterium leprae presents a complicated structure which has been studied for the last 20 years (4, 6, 8, 12), showing interesting results concerning the different components of M . leprae. Many protein constituents have been identified by immunoclectrophoretic and polyacrylamide gel electrophoretic techniques. Recently, the technique for converting bands cut from Western blots into antigen-bearing particles and testing them in lymphocyte transformation tests has been reported (7, 10, 11, 13, 14).
We have used this technique and have identified different molecular weight fractions and their behavior in the cellular response in family contacts and leprosy patients.
MATERIALS AND METHODS
Patients and contacts. Heparinized blood was obtained from patients with leprosy and their contacts. The disease was classified according to the criteria of Ridley and Jopling (16). All patients received treatment with sulfone, rifampin, and clofazimine.
Mycobacterial extracts. Soluble extract of M . leprae (MLSE) was prepared by the rupture of bacilli purified from the tissues of experimentally infected armadillos by the Draper protocol (5) with eight passes through a French pressure cell followed by centrifugation at 49,000 x g x 1 hr at 4ºC, to eliminate bacillary debris, and filtration through a Millipore membrane (pore size 0.45 µ m) (3).
MLSE fractions. Soluble extract (50 µm protein/lane) was electrophoresed on 10% polyacrylamide gels containing sodium dodecylsulfate (SDS), using a discontinuous SDS buffer system (9). The samples were diluted in a sample buffer, pH 6.8, containing 62 mM Tris-HCL, 2% SDS, 50 mM 2-mercaptoethanol and 10% glycerol, and boiled for 3 min before application on the gels. The gels were run at 25 mA (constant current) in the stacking gel and 35 mA in the separating gel until the bromophenol blue tracking dye reached the bottom of the gel. The gel was stained with 0.25% Coomassie brilliant blue. High molecular weight standard mixture SDS-6H (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) was used to determine the molecular weights (MW) of the protein bands.
The proteins in unstained preparative gels (800 µ g protein/11 cm) were electrophoretically transferred to nitrocellulose sheets (Sigma) 0.45 jmi in a Trans-blot cell (BioRad Laboratories, Richmond, California, U.S.A.) using a buffer pH 8.3 containing 25 mM Tris base, 192 mM glycine, and 20% methanol at a constant current of 100 mA (17) for 16 hr at room temperature. The nitrocellulose membranes were cut into 18 horizontal sections, selecting the strongest bands in stained vertical strips, corresponding to 120-kDa to 14-kDa MW fractions.
The strips were then solubilized as described previously (1) by incubation and intermittent mixing for 1 hr with 500 µ l DMSO, and the nitrocellulose particles were precipitated by the addition of 500 µ l sterile 0.05 M carbonate/bicarbonate buffer, pH 9.6, while vortexing the mixture vigorously. The nitrocellulose particles were suspended in 1 ml RPMI 1640 after the removal of DMSO by three washings and ccntrifugation with the same medium. Twenty µ l of each nitrocellulose fraction was added to 2 x 105 lymphocytes in 200 µ l of complete medium. Other antigens and mitogens were used as described previously (2).
Lymphocyte proliferation assays. Mononuclear cells were obtained from 30 ml of heparinized blood from leprosy patients, normal volunteers, and healthy contacts of patients as described previously (15) by centrifugation on Ficoll-Hypaque gradients (2). After 6 days of incubation, the cultures were pulsed with 1 µ Ci of tritiated methyl thymidine (specific activity 1 Ci/mole) 18 hr before harvesting, and the cells were processed for liquid scintillation counting. Mean counts per minute (cpm) of triplicate antigen-containing cultures were converted to stimulation index (SI) in relation to the nitrocellulose control culture.
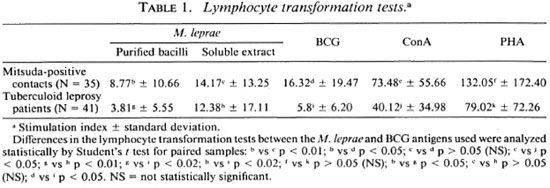
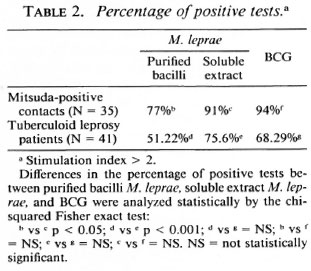
Statistical analysis. The results in Table 1 were compared using the paired Student t test and the independent Student / test for the different groups. In Table 2 the percentage ofpositive tests was compared using the chi-squared Fisher exact test and were considered significant when p was < 0.05 in both cases.
RESULTS
In our experience, the results obtained in cellular proliferation assays using MLSE have always been higher in normal volunteers, contacts of leprosy patients, and tuberculoid leprosy patients in comparison with purified M. leprae whole bacilli. Table 1 shows the details of the average proliferation obtained in a previous experiment with 35 Mitsuda-positive contacts and 41 tuberculoid leprosy patients. The results were expressed in mean ± standard deviation, using M. leprae antigens (soluble extract of M. leprae and purified bacilli) and BCG. Concanavalin A and phytohemagglutinin as mitogens were also tested. The differences were significant when we compared whole M. leprae with the MLSE. There was no significant difference between the tuberculoid patients and the contact groups using the protein extract. As shown in Table 2, the percentage of positive tests was also higher using MLSE.
Sodium-dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis of MLSE (50 µ g of protein per lane) under reducing conditions, followed by Coomassie brilliant blue staining, revealed numerous bands of different molecular weights (Fig. 1), and the most prominent transferred bands (black points beside the stained gel) were chosen for solubilization. The results of proliferation were expressed as Sis for the different fractions used (Fig. 2). Using fractionated M . leprae extract, we have observed some reactivity between 66-55, 45-29, 22-18, and at 14-kDa in the contacts and healthy volunteers (N = 13). In the two tuberculoid patients we observed some reactivity with the 36-30 and 14-kDa fractions. In the four lepromatous (LL), nonvaccinated patients, we observed lower reactivity in comparison with the three vaccinated lepromatous patients.
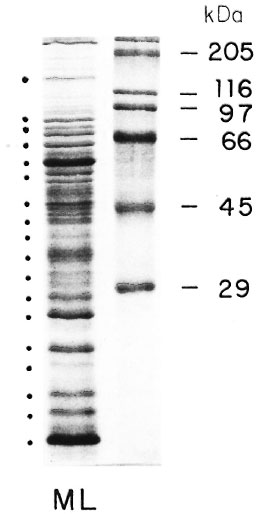
Fig. 1. SDS polyacrylamide gel electrophoresis of solube extract of M. leprae.
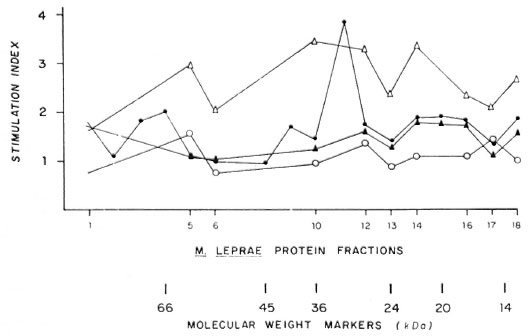
Fig. 2. Stimulation index using diferent protein fractions from MLSE in leprosy patients and Mitsuda-positive contacts. ∆- ∆= Mitsuda-positive contacts; ▲- ▲= LL vaccinated; ●- ● = HT and ○- ○ =LL not vaccinated.
DISCUSSION
Using different M. leprae antigen preparations (purified bacilli, lepromin, soluble extract prepared using the French press), we have observed that the best results have been obtained with M . leprae soluble extract and that the percentage of positivity was higher when the soluble extract was used, suggesting that the soluble extract is digested more easily by the macrophages of both groups. Similar results have been reported previously from our laboratory (15).
After electrophoretic separation and transfer to nitrocellulose membranes, we observed numerous protein bands. The 18 most prominent protein bands were selected for this study.
This technique represents a new advance over the use of whole antigen from M . leprae for the measurement of cell-mediated immunity in infected patients.
Using fractionated M. leprae extract, we observed some reactivity between the 66-55, 45-29, 22-18 and 14-kDa fractions in contacts and healthy volunteers (N = 13). In the two tuberculoid patients we observed some reactivity with the 36-30 and 14-kDa fractions but the four unvaccinated LL patients did not respond significantly to any of the fractions (SI < 1).
Other investigators have found that the 35-kDa protein is a particularly potent immunogen. This protein was precipitated by monoclonal antibody which recognizes a 35or 36-kDa protein of M . leprae and by sera obtained from patients with lepromatous leprosy (7, 14). We did serological assays with sera from unvaccinated leprosy patients (Western blots), and we obtained the two strongest bands between 24-29 and 14-kDa (data not shown).
Protective immunity did not appear to be associated with proliferation caused by any single fraction. The same observation has been reported by other groups (11, 13).
Some reactivity was observed in the vaccinated lepromatous patients when we used fractions of low molecular weight. In unvaccinated and untreated lepromatous patients, we did not observe any reaction.
In the literature we found reports of different responses using fractionated antigen. We have used M . leprae soluble extract prepared using the French pressure cell, in contrast with the other reports in the literature (7, 11, 13, 14), the pattern was very similar in the different preparations. We chose 18 fractions and the dilution used (1:10) was based on numerous preliminary assays. Although few patients were tested, we also checked the response in lymphocyte transformation tests.
Our interest now is concentrated on the low molecular weight protein associated with the cellular wall because we have found high reactivity in proliferation assays using cellwall residue in comparison with the French press and sonicated and soluble extracts (data not shown). This technique may be of practical value since comparing differences in the immunoblot profiles of polyclonal T-cell responses of infected and vaccinated or immune individuals may permit the identification of determinants capable of stimulating both T and B cells for the future design of an effective vaccine.
Acknowledgment. We wish to thank Marian Ulrich for helpful criticism of the manuscript, Jesus Sierra for elaboration of the figure, and Edward Rojas for statistical analysis. This work was supported in part by grant number 5P01 AI-26491-03 from the National Institutes of Health, Bethesda, Maryland, U.S.A.
REFERENCES
1. ABOUD-ZEID, C, FILLEY, E., STEELE, J. and ROOK. G. A. W. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro by converting lines cut from Western blots into antigen-bearing particles. J. Immunol. Methods 98(1987)5-10.
2. BOYUM, A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Invest. 21 Suppl. 97(1968)77-89.
3. CONVIT, J., ARANZAZU, N., ULRICH, M., PINARDI. M. E., REYES, O. and ALVARADO, J. Immunotherapy with a mixture of Mycobacterium leprae and BCG in different forms of leprosy and Mitsuda-negative contacts. Int. J. Lepr. 50(1982)415-424.
4. DANIEL. T. M. and JANICKI, B. W. Mycobacterial antigens: a review of their isolation, chemistry and immunological properties. Microbiol. Rev. 42(1978)84-113.
5. DRAPER, P. Protocol 1/79. Purification of M. leprae. Report of the enlarged Steering Committee meeting. Geneva, 7-8 February 1979. Annex 1. Geneva: World Health Organization, 1980. TDR/ IMMLEP-SWG(5)/80.3.
6. ELLNER, J. J., and DANIEL. T. M. Immunosuppression by mycobacterial arabinomannan. Clin. Exp. Immunol. 35(1979)250-257.
7. FILLEY, E., ABOUD-ZEID, C, WATERS, M. and ROOK, G. The use of antigen-bearing nitrocellulose particles derived from Western blots to study proliferative responses to 27 antigenic fractions from Mycobacterium leprae in patients and controls. Immunology 67(1989)75-80.
8. HUNTER. S. W., FUJIWARA, T. and BRENNAN, P. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J. Biol. Chem. 258(1982)7556-7562.
9. LAEMMLI, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(1960)680-685.
10. LAMB, J. R., O'HEHIR, R. E. and YOUNG, D. B. The use of nitrocellulose immunoblots for the analysis of antigen recognition by T lymphocytes. J. Immunol. Methods 110(1988)1-10.
11. LEE, S. P., STOCKER, N. G., GRANT, K. A., HANDZEL, Z. T., HUSSAIN, R., Mc ADAM, K. W. J. and DOCKRELL, H. M. Cellular immune responses of leprosy contacts to fractionated Mycobacterium leprae antigens. Infect. Immun. 57(1989)2475-2480.
12. MEHRA, V., BRENNAN, P. J., RADA, E.. CONVIT, J. and BLOOM, B. R. Lympohcyte suppression in leprosy by unique M. leprae glycolipid. Nature 308(1984)194-195.
13. MENDEZ-SAMPERIO, P., LAMB, J., BOTHAMLY, G., STANLEY, P., ELLIS, C. and IVANYI, J. Molecular study of the T cell repertoire in family contacts and patients with leprosy. J. Immunol. 142(1989)3599-3604.
14. MOHAGHEGHPOUR, N., MALCOLM, M . W., GELBER, R. H. and ENGLEMAN, E. G. Identification of immunostimulating protein from Mycobacterium leprae. Infect. Immun. 58(1990)703-710.
15. RADA, E., CONVIT, J., ULRICH, M., GALLINOTO, M. E. and ARANZAZU, N. Immunosuppression and cellular immunity reactions in leprosy patients treated with a mixture of Mycobacterium leprae and BCG. Int. J. Lepr. 55(1987)646-649.
16. RIDLEY, D. S., and JOPLING, W. H. Classification of leprosy according to immunity; a live-group system. Int. J. Lepr. 34(1966)255-273.
17. TOWBIN, H., STAEHELIN, T. and GORDON, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures andsome applications. Proc. Natl. Acad. Sci. U.S.A.76(1979)4350-4354.
1. M.S., Research Associate; Laboratorio de Leprología, Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
2. M.S., Biologist; Laboratorio de Leprología, Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
3. M.D., Dermatologist; Laboratorio de Leprología, Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
4. M.D., Director, Laboratorio de Leprología, Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
Received for publication on 22 May 1991.
Accepted for publication in revised form on 26 February 1992.