- Volume 60 , Number 3
- Page: 488–90
Humoral response to nerve glycolipid antigen in sera of leprosy patients
To The Editor:
Leprosy in humans is essentially a disease of the peripheral nerves. The histopathological demonstration of nerve invasion by Mycobacterium leprae or the presence of inflammatory granuloma in and around the nerve is considered essential for the diagnosis of leprosy. However, the exact mechanism underlying leprous neuritis is poorly understood. Clinical findings such as the involvement of nerves remote from the site of the infection or the absence of an etiological agent in the granulomas of tuberculoid or borderline tuberculoid leprosy cases suggest immunological involvement of the peripheral nerves as one of the possible mechanism(s). Thus, apart from investigating immune responses to M. leprae antigens in the sera of the leprosy patients, recent focus has been on the different components of the peripheral nerve as an immune target (3,4,6,8,9). Having known the potential immunological role of glycolipid antigens in the pathogenesis of the Guillain-Barre syndrome-an inflammatory, demyelinating peripheral neuropathy (10) -we attempted to detect antineural antibodies to glycolipid antigens of normal human nerves in the sera of leprosy patients as well as in the sera of patients with neural disorders other than leprosy.
Serum samples. A total of 158 serum samples-normal healthy individuals, 51; leprosy patients, 88 (typed according to Ridley Jopling classification,7); neural disorders other than leprosy, 19-were tested for antineural glycolipid antibodies at 1:200 dilution using 1.5% bovine serum albumin (BSA) in phosphate buffered saline (PBS) containing 1.5% normal rabbit serum (NRS) as a diluent.
Nerve antigen. The source of the nerve antigen was cauda equina collected at autopsy from a 26-year-old female with no history of any neurological disorder. The connective tissue and epineurium were removed, and the tissue stored at - 70ºC until further use. Thin sections (approximately 15-µm thick) were cut using a freezing microtome (cryostat) and then processed for glycolipid antigen extraction according to the method of Ariga, et al.(1). The lipid content was determined and 1 µg per 50µl was used for coating in an ELISA system.
ELISA procedure. The ELISA procedure was essentially that of Ilyas, et al.(5) with minor modifications as follows: one half of the wells of the microtitcr polystyrene plates (Immulon 2; Dynatech Inc., Chantilly, Virginia, U.S.A.) were coated with 50µl of methanol; the remaining half were coated with 50µl of nerve glycolipid antigen (NGA) in methanol for 18 hr at room temperature (RT). After blocking the unrcactcd sites with 3% BSA in PBS for 2 hr at RT, 100µl of the diluted serum samples, as described earlier, were added in duplicate to the methanol- as well as the antigen-coated wells. The plates were incubated for 5 hr at RT, drained, washed four times with PBS and peroxidase-labeled rabbit antihuman IgM (1:1000 in 10% NRS; Dakopatts, Glostrup, Denmark) was added. After incubation for 1 hr at 37ºC, the wells were drained, washed four times with PBS, and the enzyme-linked immune complex was quan- titated using H2O2-O-phenylene diamine as a substrate (0.4 mg/ml in citrate phosphate buffer, pH 5.0) at 490 nm using a Minireader II (Dynatech). The difference in the optical density (OD) obtained with the antigen-coated wells and methanol-coated wells was calculated with normal as well as patient sera. The cut-off value (∆ OD at 490 mm) of 0.15 (0.035 ± 0.039 S.D., normal sera) was used for screening the sera positive.
Results. The overall percent positivity obtained with the 88 leprosy patients evaluated was 23.86% (21/88), and all of them belonged to the lepromatous or borderline lepromatous or purely borderline type of leprosy classification, thus giving a percent positivity of 31.3 (21/67) in the lepromatous leprosy group. The antibodies to NGA were absent in tuberculoid-borderline tuberculoid (TT-BT) type of leprosy as well as in 25 patients with neural disorders other than leprosy (Fig. 1). An attempt was made to correlate the presence of anti-PGL-I an tibodics with anti-NGA antibodies in these patients (Fig. 2). As is evident, an overall poor correlation (r = 0.37) was obtained between the two types of antibodies. This observed discordance could be due to the smaller size and heterogeneous group of patients [tuberculoid to lepromatous (TT to LL) type] with varying treatment status, from no treatment to monodrug therapy/ multidrug therapy for varying periods of times. It is worthwhile to note that in our series the untreated group tends to show lower/negligible immunoreactivity to nerve lipid antigen in spite of having a high bacterial load, while the treated group of patients tends to have higher levels of immunoreactivity. The absence of antibodies to NGA in the early untreated leprosy cases suggests the possible role of cytoplasmic antigen (released during treatment) in nerve reactions as postulated by Barnetson, et al.(2).
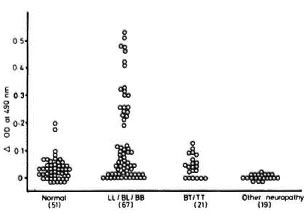
Fig. 1. Reactivity to nerve glycolipid antigen of sera from healthy normal individuals and patients with various types of leprosy along with other neuropathies. LL = Lepromatous; BL = borderline lepromatous; BB= borderline; BT = borderline tuberculoid; TT = tuberculoid leprosy.
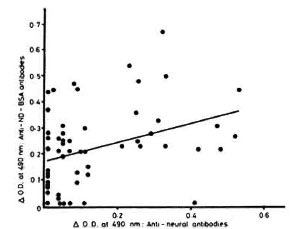
Fig. 2. A scatter diagram showing the correlation between anti-natural disaccharide (of phenolic glycolipid-I) bovine serum albumin (ND-BSA) antibody and anti-nerve glycolipid antigen-antibody reactivity in sera from leprosy patients.
Our efforts in demonstrating the presence of antibodies to whole nerve sonicate in the sera of leprosy patients using SDS-PAGE electrophoresis showed a pattern comparable to that seen with normal sera. Similar results of equal binding of sera from normal and leprosy patients to peripheral nerve has been reported by Ghaswala, et al.(4).
The studies reported in this communication indicate that a specific nerve glycolipid component appears to be involved in the immune mechanism. Further characterization of the antigen along with its utility for monitoring the course of the disease is in progress.
- Rekha V. Sahasrabudhe, B. Pharm.
Acworth Leprosy Hospital Research Society
- Subhada R. Dandekar, M.Sc.
Damayanti H. Shah, Ph.D.
Radiation Medicine Center (BARC)
Tata Memorial Center Annex
Parel, Bombay 400012, India
- Subhada S. Pandya, M.B.B.S.
Sharad S. Naik, M.Sc.
Evelyn Sequeira, B.Sc.
Acworth Leprosy Hospital Research Society
Wadala, Bombay 400031, India
- R. Ganapati, M.B.B.S., D.V.D.
Bombay Leprosy Project
Sion-Chunabhatti
Bombay 400022, India
REFERENCES
1. ARIGA, T.. KOHRIYAMA, T. and FREDDO, L. Characterization of sulphated glucoronic acid containing glycolipids reacting with IgM M-proteins in patients with neuropathy. J. Biol. Chem. 262(1987)848-853.
2. BARNETSON, R. ST.C , BJUNE, G., PEARSON. J. M. H. and KRONVALL, G. Cell mediated and humoral immunity in "reversal reactions." Int. J. Lepr. 44(1976)267-274.
3. EUSTIS-TURF, E. P., BENJAMINS, J. A. and LEFFORD, M. J. Characterization of anti-neural antibodies in the sera of leprosy patients. J. Neuroimmunol. 10(1986)313-330.
4. GHASWALA, P. S., MISTRY, N. F. and ANTIA, N. H. Serum antibodies of normals and leprosy patients show equal binding to peripheral nerve. Int. J. Lepr. 57(1989)690-692.
5. ILYAS, A. A., QUARLES, R. H., MACINTOSH, T. D., DOBERSON, M. J., TRAPP, B. D., DALAKAS, M. C. and BRADY, R. O. IgM in human neuropathy related to paraproteinemia binds to a carbohydrate determinant in the myelin associated glycoprotein and to a ganglioside. Proc. Natl. Acad. Sci. U.S.A. 81(1984)1225-1229.
6. PRAKASH, O., SREEVATSA, P. and SENGUPTA, U. An attempt to demonstrate anti-nerve antibodies in leprosy sera using rabbit nerve as an antigen. Int. J. Lepr. 58(1990)129-130.
7. RIDLEY, D. F. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
8. THOMAS, B. M. and MUKHERJEE, R. Antineural antibodies in sera of leprosy patients. Clin. Immunol. Immunopathol. 57(1990)420-429.
9. VEMURI, N. and MUKHERJEE, R. Immunoreactivity of nerve lipid antigens in leprosy. J. Clin. Lab. Anim. 5(1991)157-161.
10. Yu, R. K., ARIGA, T., KOHRIYAMA, T., KUSUNOKI, S., MEDA, Y. and MIYATANI, N. Autoimmune mechanisms in peripheral neuropathies. Ann. Neurol. 27Suppl.(1990)S30-S35.
Reprints requests to Dr. Shah.