- Volume 58 , Number 2
- Page: 347–52
A quantitative study of the relationship between systemic and histological parameters ot immunity in individual leprosy patients
ABSTRACT
A group of 52 untreated leprosy patients were examined to determine the relationship between local and systemic immunological parameters across the clinico-patho-logical spectrum. The Ridley-Jopling classification, bacterial index (131), and granuloma fraction (GF) were assessed in biopsies f rom 40 cases. The densities of apop-toses, mitoses, and plasma cells were also measured. Systemic immunity to mycobacteria was assessed by skin tests with leprosin A and PPD, and by measurement of the serum antibody responses to Mycobacterium leprae, M. tuberculosis, and M. scrofulaceum. The serum responses to phenolic glycolipid-I (PGL-I) of M. leprae was assessed using a glycoconjugate which mimics an immunodominant epitope.The serum antibody levels and skin test results showed the expected inverse relationship. The BI within lesions showed an inverse correlation with the skin test results, but none of the other histological parameters studied showed a significant relationship with the other measurements of systemic immunity. Our findings suggest that the inverse relationship between delayedtype hypersensitivity and humoral immunity in leprosy patients, which is strong in groups of patients across the leprosy spectrum, is less strong in individual patients than is often thought. The lack of correlation of many histological and systemic parameters suggests that local factors modulate systemic immunity in the pathogenesis of leprosy lesions.
RÉSUMÉ
On a examiné un groupe de 52 malades atteints de lèpre et non traités, en vue de déterminer la relation existant entre les paramètres immunologiques locaux et systémiques, d'un bout à l'autre du spectre clinico-pathologique. Sur les biopsies provenant de 40 cas, on a procédé à la classification de Ridlcy-Jopling, à l'évaluation de l'index bactériologique (BI), et à la détermination de la fraction granulomateuse (GF). On a également mesuré les densités des apoptoses, des mitoses, et des cellules plasmatiques. On a estime l'immunité systémique aux mycobactérics au moyen d'épreuves cutanées par la léprosine A et par le PPD, de même qu'en mesurant les réponses du sérum en anticorps contre Mycobaclerium leprae, M. tuberculosis, et M. scrofulaceum. Les réponses sériques à l'antigène phénoglycolipidique-I (PGL-I) de M. leprae ont été déterminées en utilisant un glycoconjugué qui simule l'épitope immunodominant.Les taux d'anticorps sériques, de même que les résultats de l'épreuve cutanée, ont montré, ainsi qu'on pouvait s'y attendre, une relation inversée. L'Index Bactériologique dans les lésions a révélé une corrélation négative avec les résultats de l'épreuve cutanée; toutefois, aucun des autres paramètres histologiques étudiés n'a montré une relation significative avec les autres déterminations de l'immunité systémique. Ces observations suggèrent que la relation inversée entre l'hypersensibilité de type retardé et l'immunité humorale chez les malades de la lèpre, qui est très marquée dans des groupes de malades tout au long du spectre de la lèpre, est cependant chez des malades pris individuellement, moins prononcée qu'on ne le pense souvent. L'absence de corrélation entre un bon nombre des paramètres histologiques et systémiques suggère que des facteurs locaux modulent l'immunité systémique au cours de la pathogenèse des lésions de la lèpre.
RESUMEN
Se estudió un grupo de 52 pacientes con lepra sin tratamiento para determinar la relación entre inmunidad local y sistémica a lo largo del espectro clínico-patológico de la enfermedad. Se estableció la clasificación (Ridley-Jopling). el índice bacteriano (IB) y el tamaño de la fracción granuloma (FG) en las biopsias de piel de 40 pacientes. También se midió el grado de apoptosis y de mitosis, y la densidad de células plasmáticas. La inmunidad sistémica contra la microbac-teria se midió por las pruebas dérmicas con leprosina y con PPD y por medición de los niveles séricos de anticuerpos contra Mycobacterium leprae, M. tuberculosis, y M. scrofulaceum. Las respuestas séricas contra el glicolipido fenólico-1 (GLF-1) del M. leprae se midieron usando un glicoconjugado que mimetiza un epítope inmunodominante.Los resultados sobre los niveles de anticuerpos séricos y aquellos sobre las pruebas dérmicas mostraron la relación inversa esperada. Aunque el IB dentro de las lesiones mostró una correlación inversa en los resultados de las pruebas dérmicas, ninguno de los otros parámetros estudiados mostró una relación significativa con las otras mediciones de la inmunidad sistémica. Nuestros hallazgos suqieren que la relación inversa entre la inmunidad retardada y la inmunidad humoral en los pacientes con lepra, la cual es marcada entre los diferentes grupos del espectro de la enfermedad, es menos marcada de lo que se piensa cuando se analizan los pacientes individuales. La falta de correlación de muchos parámetros histológicos y sistémicos, sugiere que en la patogénesis de las lesiones de la lepra, la inmunidad sistémica es modulada por factores locales.
Despite the large volume of published work which suggests a broad relationship between cell-mediated immunity in the lesions and systemic immunity to Mycobacterium leprae, there have been few recent studies of the relationship between local (lesional) and systemic immunological parameters in individual leprosy patients. One such study by Sehgal, et al. (13) examined the degree of agreement of clinical, bacteriological, histological, and immunological (lepromin test) parameters. While their results provide some support for the observations which form the basis of the Ridley-Jopling classification of leprosy (10,12), good correlation between all four of the parameters studied was seen in only 44% of their patients.
Morphometric analysis of leprosy lesions allows the extraction of quantitative data from these lesions and has proved useful for the assessment of the granuloma fraction (GF), and parameters of cell turnover (3,7). In this study, we have examined these histological measurements, together with clinical and immunological parameters, in a group of untreated leprosy patients to determine whether the apparent relationships between these variables across the Ridley-Jopling classification can be applied to individuals.
MATERIALS AND METHODS
Subjects. The study was performed prospectively and included 52 leprosy patients presenting during a population survey or by self-referral to the HEED Leprosy Hospital, Kamalganj, Bangladesh. All of the patients were newly diagnosed and none had received any treatment. The average age of the patients was 33.1 years (range 10-60 years) with a male:female ratio of 1:0.86. All of the subjects gave verbal informed consent for the tests, the results of which were made available to the local leprosy control team.
Clinical tests. All of the subjects underwent clinical examination, and skin tests for delayed hypersensitivity to mycobacterial antigens were performed by giving each subject intradermal injections of tuberculin, purified protein derivative (PPD) (1:1000 dilution; Evans Medical, Beaconsfield, England) and Rees' skin test antigen (leprosin A; Batch CD19) on the volar aspect of the right and left forearms, respectively. The results were expressed as the mean diameter of the area of induration at 72 hr, measured using the ball-point method (15). The nutritional status of each subject was assessed clinically by assessing the skin-fold thickness of the upper arm using Harpenden skinfold callipers (British Indicators Ltd., St. Albans, England) and by mid-arm circumference (cm).
Serum samples were obtained from each subject by venipuncture using sterile disposable equipment and preserved with sodium azide (0.5 mg/ml final concentration). The samples were refrigerated until they could be transported to Dundee, Scotland, for analysis.
Skin biopsies. Skin biopsies were taken from the edge of an established skin lesion in 40 of the 52 patients using sterile disposable 4-mm skin biopsy punches (Stiefel Laboratories, Slough, England) (3). Following fixation in 4% buffered formaldehyde, each biopsy was bisected and embedded in paraffin wax. Sections of 5 μm were stained with hematoxylin and cosin, by the Wade-Fite method for acid-fast bacteria, and with methyl green pyronin for plasma cell enumeration.
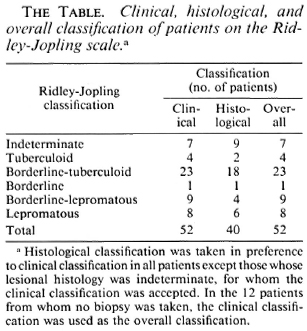
Each biopsy was classified on the Ridley-Jopling scale (12). The histological classification was taken in preference to the clinical classification in all patients except those whose lesional histology was indeterminate, when the clinical classification was accepted. In the 12 patients for whom no biopsy was taken, the clinical classification was used as the overall classification. The histological, clinical, and overall classification of the patients included in the study are shown in The Table.
Histometry. The bacterial index (BI) was estimated semi-quantitatively using the method described by Ridley and Hilson (11). The granuloma fraction (GF) was measured by planimetry with methods described previously (3,4,7). Plasma cell counts were performed on nonoverlapping fields at x400 magnification by scanning the whole section using a 25-square eyepiece grid. Apoptotic and mitotic densities were estimated similarly (5). The results were expressed as the plasma cell density in cells per mm2 granuloma.
ELISA. Whole γ-irradiated mycobacteria (M. leprae, M. tuberculosis H37Rv and M. scrofulaceum) were dried onto gelatine-coated polystyrene microtiter plates for use in a whole mycobacterial ELISA (6). After blocking with 5% normal goat serum (NGS), the wells were incubated with test sera at a 1:200 dilution, washed, and incubated with polyclonal goat anti-human immunoglobulin conjugated to horseradish peroxidase (Sigma, Poole, U.K.) at a 1:1000 dilution. Fresh 0.4 mg/ml o-phenylene diamine with hydrogen peroxide was used as the peroxidase substrate. The reaction was stopped with 2.5 N sulfuric acid and read at 490 nm using a Dynatech MR580 MicroELISA au-torcader. The assays for antibody against M. leprae were performed for three immunoglobulin subclasses (IgG, IgM, IgA); assays for antibody against M. tuberculosis and M. scrofulaceum were performed using a second antibody reacting with all subclasses.
The samples were also screened in an ELISA developed by Brett, et al. (1), using a bovine serum albumin (BSA) glycocon-jugate (BSA-C) which mimics a major epitope of the phenolic glycolipid-I (PGL-I) of M. leprae. Conjugate 11 (1) was kindly provided by Dr. R. J. W. Rees, National Institute for Medical Research, London, U.K. This glycoconjugate consists of the intact terminal disaccharide of the natural antigen linked to BSA by reductive amination (1). The ELISA was modified slightly by the use of 5% NGS in the blocking stage for scrum assays. (6).
Data analysis. The results were analyzed on a microcomputer using nonparametric methods (Statgraphics, STSC, California, U.S.A.), since the ELISA and histological results did not conform to a normal distribution. In all cases the correlation coefficients (r) quoted in the text are Spearman rank correlation coefficients derived from the raw data: p values are indicated by one asterisk (p < 0.05) or by two asterisks (p < 0.005).
RESULTS
Relationship between individual histological parameters (N = 40). There is the expected relationship between the BI and overall classification on the Ridley-Jopling scale, but none between the GF and histological grade. However, the GF docs correlate with the BI (r = 0.49**), apoptotic density (r = 0.51**), and mitotic density (r = 0.51**). The relationship between apop-totic density and mitotic density is weak (r = 0.39*), suggesting that mitosis is of limited importance in maintaining the cell population size in leprosy granulomas (3,5). The plasma cell density within the lesions does not correlate with any of the other histological parameters.
Relationship between individual parameters of systemic cell-mediated and humoral immunity (N = 52). There are significant negative correlations between the leprosin A skin test results and the serum antibody response to both M. tuberculosis (r = - 0.47*) and M. scrofulaceum (r = -0.53**), consistent with an inverse relationship between cell-mediated and humoral immunity to common mycobacterial antigens. However, there is no correlation between the leprosin A results and the scrum antibody response to M. leprae (IgG, IgM or IgA), suggesting that this relationship may not extend to M. leprae-specific antigens. A weaker negative correlation between the leprosin A results and serum IgM response to BSA glycoconjugatc (r = -0.31*) is also present. The PPD skin test results do not correlate with any of the serum antibody responses.
Relationship between histology and systemic immunity (N = 40). The predicted overall relationship between the Ridley-Jopling classification and the results of the scrum ELISAs are present: the strongest being that with the serum IgM response to BSA glycoconjugatc (Fig. 1), although the scrum IgG response to whole M. leprae is less impressive (Fig. 2). The BI is positively correlated with the scrum ELISAs for IgM (r = 0.48**) and IgG against BSA glycoconjugate (r = 0.39*) and antibody against whole M. tuberculosis (r = 0.43*), but there is no correlation between IgG or IgM responses to whole M. leprae and the BI. Positive leprosin A responses (> 5 mm diameter induration) were seen in 12 of the 20 tuberculoid (TT/BT) patients, in 2 of 9 indeterminate histology patients, and in 1 of the 11 multibacillary patients biopsied, a borderline-lepromatous case. There is negative correlation between the BI and the results of the leprosin A (r = -0.44*) and PPD skin tests (r = -0.57**). There are no significant relationships between apoptotic or mitotic density and the results of the immunological measurements. There is no relationship between the plasma cell density and the ELISA results, except for a weak negative correlation with the serum IgG response to whole M. leprae (r = -0.39*). This suggests that the plasma cells within the lesion do not make a large contribution to the quantity of circulating antibody. Neither nutritional parameter showed any correlation with the ELISA or skin test results.
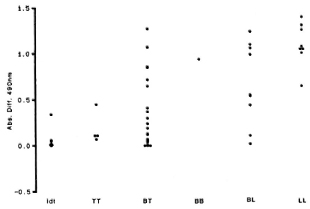
Fig. 1. Relationship of the serum IgM response to BSAglycoconjugate with Ridley-Jopling classification, showing clear gradation of this antibody response across the leprosy spectrum with the highest levels in LL patients.

Fig. 2. Relationship of the serum IgG response to whole γ-irradiated M. leprae with Ridley-Jopling classification, showing high levels among some patients of all classifications.
DISCUSSION
In this study, the concept that leprosy is a disease which exhibits a spectrum of closely linked immunological, histological, and clinical parameters has been tested for its relevance to individual patients by measuring representative parameters which might be expected to show some relationship to each other. There are broad associations between the parameters measured, but the generally accepted model of an inverse relationship between cell-mediated and humoral immunity does not apply strictly to individual patients, in whom the response is evidently much more complex.
The paucity of significant correlations between skin test and ELISA results is particularly striking, since it suggests that de-layed-type hypersensitivity and humoral immunity may have only a weak inverse relationship and that this may be strongest for common mycobacterial antigens. Studies of the antigens involved in the humoral immune response to M. leprae and recent studies of the antigens recognized by T lymphocytes cloned from leprosy patients have shown that humoral and cell-mediated immune responses in leprosy are the end result of divergent responses to different epitopes (2,8). Our results demonstrate that this divergence between humoral and cell-mediated immune responses is reflected in leprosy patients. The cell-mediated immune responses which dominate in tuberculoid leprosy are unlikely to be linked with the antibody levels upon which serodiagnostic tests for leprosy depend, which explains the relative inability of specific serological tests to detect paucibacillary leprosy (6,12,14).
Despite the apparent lack of correlation between the humoral and cell-mediated immune responses in individual leprosy patients, it is evident that there is a clinico-pathological spectrum in leprosy which is a useful model of the disease, even if the relationships between the various components of the spectrum do not apply strictly to individual cases. While positive leprosin A results are more common in paucibacillary patients, positive results can occasionally be obtained in multibacillary patients, limiting the usefulness of this skin test for patient classification.
The relationship between the histological appearance of the skin lesions (Ridley-Jopling classification) and the immunological parameters studied is evident in individual leprosy patients. A comparison of the skin test and ELISA results with the histological measurements suggests that there is a strong relationship between cell-mediated immunity and lesional histology. Antibody levels to M. leprae are less strongly related to lesional histology, and are independent of the plasma cell density within the lesion. Local factors which could modulate the systemic cell-mediated response to M. leprae include the age of the lesion and site-dependent differences in cell content (for instance, Lang-erhans' cell or mast cell numbers), antigen exposure, or blood flow. Local factors appear to modulate the expression of systemic immunity in the pathogenesis of the skin lesion in leprosy. This may explain histological differences between lesions which seem to occur in some patients (7,13).
Acknowledgments. We are grateful to Dr. I. H. Cochrane. Dr. R. Hart, Dr. K. Hatano, and Dr. M. S. Islam for their assistance in Bangladesh. The mycobacteria and BSA glycoconjugatc for the ELISAs were kindly provided by Dr. R. J. W. Rees (Division of Communicable Diseases. Clinical Research Centre, Harrow). We also wish to thank Mr. G. Coghill for technical assistance and Mr. R. Fawkes for preparation of the illustrations. The visit to Bangladesh by IAC was funded by the award of the Becton-Dickinson traveling scholarship by the Royal College of Pathologists, and the laboratory studies were supported by LEPRA.
REFERENCES
1. Brett, S. J., Payne, S. N., Gigg, J., Burgess, P. and Gigg. R. Use of synthetic glycoconjugates containing the Mycobacterium leprae specific and immunodominant epitope of phenolic glycolipid I in the serology of leprosy. Clin. Exp. Immunol. 64(1986)476-483.
2. Converse, P. J., Ottenhoff, T. H. M., Ehrenberg, J. P. and Kiessling, R. The repertoire of mycobacterial antigens recognised by peripheral blood cells and sera of healthy leprosy patient contacts. (Abstract) Int. J. Lepr. 55(1987)779-781.
3. Cree, I. A., Gardiner, C. A., Beck, J. S. and Mehta, J. Studies of cell death (apoptosis) and cell division in leprosy granulomas. Int. J. Lepr. 54(1986)607-613.
4. Cree, I. A., McDougall, A. C, Coghill, G. and Beck. J. S. Quantitation of the granuloma fraction in leprosy skin biopsies by planimetry. Int. J. Lepr. 53(1985)582-586.
5. Cree, I. A.. Nuruhai, S., Milne, G. and Beck, J. S. Cell death in granulomata: the role of apoptosis. J. Clin. Pathol. 40(1987)1314-1319.
6. Cree, I. A., Smith, W. C. and Beck, J. S. Serum antibody responses in leprosy patients and their contacts. Lcpr. Rev. 59(1988)317-328.
7. CrEe, I. A., Srinivasan, T., Krishnan, S. A. R., Gardiner, C. A., Mehta, J., Fisher, C. A. H. and Beck. J. S. Reproducibility of histology in leprosy lesions. Int. J. Lepr. 56(1988)296-301.
8. Lamb. j. R.. Ivanyi, J., Rees. A., Young, R. A. and Young, D. B. The identification of T coll epitopes in Mycobacterium tuberculosis using human T lymphocyte clones. Lepr. Rev. 57 Suppl. 2(1986)131-137.
9. Melsom, R. Serodiagnosis of leprosy: the past, the present and some prospects for the future. Int. j. Lepr. 51(1983)235-252.
10. Ridley, D. S. Histological classification and the immunological spectrum of leprosy. Bull. WHO 51(1974)451-465.
11. Ridley, D. S. and Hilson, G. R. F. A logarithmic index of bacilli in biopsies. Int. j. Lepr. 35(1967)184-186.
12. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a live-group system. Int. J. Lepr. 34(1966)255-273.
13. Sehgal, v. n., Koranne. R. v., Sehgal, S., Beo-iilr, P. C. and Siiarma, V. K. Correlation of morphological, bacteriological, histopathological and immunological features of leprosy. A double-blind study. J. Dermatol. 12(1985)243-250.
14. Serological tests for leprosy. (Editorial) Lancet 1(1986)533-535.
15. Sokal, J. E. Measurement of delayed skin test responses. N. Engl. J. Med. 293(1975)501-502.
1. MB., Ph.D., M.R.C.Path., Senior Lecturer, Department of Pathology; Nine-wells Hospital and Medical School, Dundee DD1 9SY, Scotland, U.K.
2. M.D., M.P.H., M.F.C.M., Hon. Senior Lecturer. Department of Community Medicine; Nine-wells Hospital and Medical School, Dundee DD1 9SY, Scotland, U.K.
3. M.D., F.R.C.Path., F.R.S.E., Professor, Department of Pathology, Nine-wells Hospital and Medical School, Dundee DD1 9SY, Scotland, U.K.
Received for publication on 10 July 1989.
Accepted for publication in revised form on 17 January 1990.