- Volume 58 , Number 1
- Page: 123–6
Screening of anti-M leprae antibodies in the blood samples eluted from filter paper blood blots
To the Editor:
The conventional method of blood collection by venipuncture for the serodiagnosis of leprosy does not suit field conditions for the reasons that a) these samples have to be transported from field areas to the reference laboratory under controlled temperature to maintain stability and b) the procedure of collection of venous blood is not uniformly acceptable to the field population. We report here a methodology for an easy and simple method of collection of blood samples from a population in the field and then transport of the samples to the reference laboratory for assay. This report also describes a comparative evaluation of the data of conventionally collected serum and blood collected on filter papers for the presence of specific antibodies using a monoclonal-antibody-based immunoradiometric assay by the antibody competition test.
Blood from 86 leprosy patients-28 from Central JALMA Institute for Leprosy (CJIL), Agra, and 58 from Sacred Heart Leprosy Centre (SHLC), Kumbakonam-77 contacts (73 from SHLC and 4 from CJIL) and 10 healthy laboratory volunteers (CJIL) was used for this study. The patients included 47 with borderline lepromatous/lepromatous (BL/LL) leprosy, 22 with tuberculoid/borderline tuberculoid (TT/BT) leprosy, 5 with midborderline (BB) leprosy, 4 with primary neuritic leprosy, and 8 with indeterminate leprosy. The classification of leprosy was clinical, based on Ridley-Jopling criteria (4).
The method of Burgess, et al. (1) was followed for the finger-prick blood sample collection. Four drops (50 ×l) of blood were collected from each individual on two (1.25" × 3" size) separate Whatman no. 3 filter papers (Whatman International Ltd., Maidstone, U.K.). These blood samples taken on the filter paper (blood blots) at CJIL were air dried at room temperature. One blot of each sample was stored at 4ºC, the other blot at 40ºC for 2 weeks. Venous blood samples were also collected from CJIL subjects for initial standardization of the assay.
The blood sample from each SHLC subject was collected only on filter paper. These samples were air dried, coded, and dispatched by surface mail to CJIL, Agra, for antibody assay.
A metal punch of 16 mm diameter was used to cut the blood blots from the center. The filter paper pieces were soaked individually with 250 ×l of phosphate buffered saline (PBS), pH 7.4, in wells of a 24-well tissue culture plate. Elution with 250 ×l PBS for each 16 mm blood blot disc gave a value equivalent to 1:10 dilution of serum at 50% hematocrit value (1).
The monoclonal antibody (MAb) ML04, which binds to a highly immunogenic epitope on the 35-kDa soluble protein antigen of Mycobacterium leprae (3), was labeled with Na 125I (BARC, Bombay, India) by the iodogen method (2).
In this antibody competition test, a flexible 96-well microtiter plate (Dynatech, Cambridge, Massachusetts, U.S.A.) was coated with 50 ×l/well (50 ×g/ml) of M. leprae antigen by overnight incubation at 4ºC. Unbound M. leprae antigen was later removed by washing with PBS. The wells were blocked with 100 ×l/well of 2% bovine serum albumin (BSA) (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) in PBS for 2 hr at room temperature. Unbound BSA was removed by dumping the plate followed by patting on tissue paper. Twenty-five ×l of each eluate sample was added to the wells in duplicate and incubated for 1 hr at 37ºC in a moist chamber, followed by the addition of 25 ×l of 125I-ML04 (cpm 80,000-100,000) to each well. Further incubation was carried out for 2 hr at 37ºC. The whole plate was then washed three times with PBS and blotted dry. The radioactivity was measured in a gamma counter (NE 1600, U.K.). The eluates from normal individuals had not shown inhibition of binding of 125I-ML04 by > 40%; hence, eluates showing >50% inhibition were considered to be antibody positive. The antibody titer was calculated on the basis of dilution of the sample at which it shows 50% inhibition, using the formula:

100% binding being the binding of 125I-ML04 directly to the antigen-coated well after blocking with 2% BSA in PBS ( 1000-2500 cpm). The assays were don without the knowledge of the source of blood (serum or blot) in all cases.
1000-2500 cpm). The assays were don without the knowledge of the source of blood (serum or blot) in all cases.
It was found that the percent inhibitions obtained from serum samples and corresponding blood blots from the 42 CJIL subjects were very similar. It was also found that the storage temperature of the blood blot (4ºC or 40ºC for 2 weeks) did not affect the results, and for both temperatures of storage the correlation in the antibody-positive cases was as high as 0.97 to 0.98 (Fig. 1).
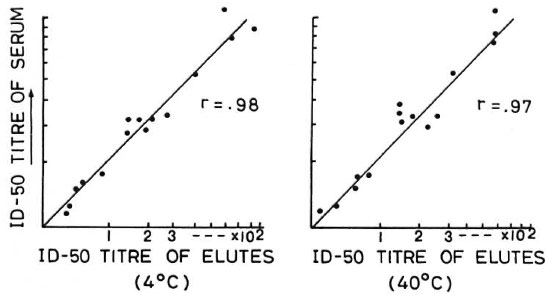
Fig. 1. Comparison of titers of sera and eluates of corresponding blood blots stored at 4°C and 40°C byantibody competition test.
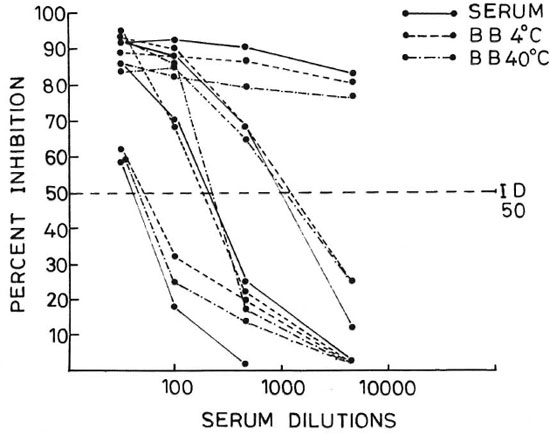
The antibody titers were calculated on the basis of dilution of the sample at which it shows 50% inhibition (ID-50). Representative inhibitory patterns for serum and blood blots stored at 4ºC and 40ºC from individuals showing low, moderate and high antibody titers are shown in Figure 2. The correlation between antibody titer obtained in sera and corresponding blood blots is shown in The Table. It can be seen that all serum samples which were positive for specific antibodies were also positive in their corresponding eluates obtained from blood blots. Likewise, all of the antibody-negative serum samples were also negative in their blood-blot eluates. The titer values for blood blots stored at 4ºC and 40ºC were also nearly the same in all cases, except for one case of LL with erythema nodosum leprosum (ENL) for whom the values were 1100 in serum, 900 in blood blot stored at 4ºC, and 700 in blood blot stored at 40ºC.
The results of blood blots sent to us by mail from SHLC (2000 km from Agra) have shown that all of the 27 BL/LL cases, and all of the 5 BB cases as well as 6 of the 14 TT/BT cases were antibody positive.
These results demonstrate that an antibody assay is possible from blood samples collected on Whatman no. 3 filter paper, and that the results obtained are comparable to those collected by venipuncture. The percentage of positivity in blood blots obtained from different types of patients and contacts from SHLC is well in agreement with our earlier positivity rates observed with serum samples (5), indicating the reliability of this method of blood collection. As will be evident, for use in the field, this method of blood collection is very much less expensive than venipuncture because it docs not require other peripherals such as disposable syringes, needles, test tubes, storage vials, and deep freezers. Since the field worker can collect and store a large number of samples using this method, this procedure will be highly suitable for largescale epidemiological studies. We have found that once the blood sample was dried on filter paper and kept dry, it remained preserved (even up to 1 year) until it was reconstituted, thus averting the common problem of contamination. We have also found that the extreme storage temperature of 40ºC for 2 weeks did not affect the antibody levels of the samples. It appears, therefore, that this is a reliable method which can be used despite seasonal variations of temperature.
- Shripad A. Patil, Ph.D.
Department of Immunology
Central JALMA Institute for Leprosy (ICMR)
Taj Ganj
Agra 282001, India
- Gopal Ramu, M.D.
Sacred Heart Leprosy Center
Kumbakonam, South India
- S. Sinha, Ph.D.
U. Senguta, Ph.D.
Department of Immunology
Central JALMA Institute for Leprosy (IMCR)
Taj Ganj
Agra 282001, India
Acknowledgments. We thank Dr. R. J. W. Rees, IMMLEP (WHO) Hank, London, and Dr. J. Ivanyi, Medical Research Council, Hammersmith Hospital, London, for providing us with soluble M. leprae antigen and globulin fraction of monoclonal antibodies, respectively. We are also grateful to Dr. H. Srinivasan, Director, CJIL, Agra, for critical review of the manuscript and to Mr. R. K. Saxena for statistical analysis. The secretarial assistance by Mr. Anil Kumar Chopra, technical assistance by P. N. Sharma, Kuldeep, Malkhan, and photography by Hari Om Agarwal is acknowledged. Part of the work was supported by ad hoc grants from the Indian Council of Medical Research (vide scheme 1/8/82).
REFERENCES
1. BURGESS, P. J., FINE, P. E. M., PONNIGHAUS, J. M. and DRAPER, C. C. Serological tests in leprosy. The sensitivity, specificity and predictive value of ELISA tests based on phenolic glycolipid antigens, and the implications for their use in epidemiological studies. Epidemiol. Infect. 101(1988)159-171.
2. FRAKER, P. J. and SPECK, J. C, JR. Protein and cell membrane iodination with a sparingly soluble chloramide 1,3,4,6-tetrachloro-3a,6a-diphenylglycoluril. Biochem. Biophys. Res. Commun. 80(1978)849-857.
3. IVANYI, J., SINHA, S., ASTON, R., CUSSELL, D., KEEN, M. and SENGUPTA, U. Definition of species specific and cross-reactive antigenic determinants of Mycobacterium leprae using monoclonal antibodies. Clin. Exp. Immunol. 52(1983)528-536.
4. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
5. SINHA, S., SENGUPTA, U., RAMU, G. and IVANYI, J. Serological survey of leprosy and control subjects by a monoclonal antibody based immunoassay. Int. J. Lepr. 53(1985)33-38.