- Volume 57 , Number 3
- Page: 615–21
Effect of chemotherapy on viability of Mycobacterium leprae as determined by ATP content, morphological index and FDA-EB fluorescent staining
ABSTRACT
Viable bacterial populations were estimated in bacilli purified f rom 105 biopsies f rom 40 untreated and 65 multibacillary leprosy patients treated with multidrug therapy (MDT) for varying periods. The bacilli were purified and viability was determined by ATP content, morphological index (MI), and fluorescein diacetate-ethidium bromide (FDA-EB) staining. Viable populations were calculated, taking 3.58 x 10-15 g/solid bacillus as the mean ATP content of a viable unit of Mycobacterium leprae. The proportion of viable bacilli was also estimated in the same specimens using solid-staining (MI) and green-staining bacilli by the FDA-EB method. In the untreated cases, the positive viability by ATP assay was 100%, 92% by MI, and 100% by FDA-EB. ATP content per solid bacillus was relatively constant, which was not the case with ATP content per green-staining bacillus. While the MI was zero in all cases, viable bacilli could still be detected by ATP estimations in 5 of the 32 (16%) patients after 2 years of MDT and in 1 of the 20 (5%) patients after 3 years of MDT. No viable bacilli could be detected even by this method beyond 3 years of MDT. On the other hand, green-staining bacilli were demonstrable in 7/32 (22%) of cases after 2 years of MDT, 2/20 (10%) after 3 years of MDT, and 1/13 (8%) after more than 3 years of treatment, indicating that the FDA-EB staining and ATP assay did not detect the same populations. A determination of the ATP content of M. leprae could be used as a reliable and sensitive tool for determining viability of the bacilli.RÉSUMÉ
Dans des biopsies provenant, pour 105 d'entre elles, de malades de la lèpre non traités, et pour les 65 autres de sujets multibacillaires traités par la polychimiothérapie, pendant des durées variables, on a estimé les populations de bactéries viables parmi des bacilles purifiés. On a déterminé la viabilité des bacilles purifiés en se basant sur le contenu en ATP, l'index morphologique (IM), et la coloration par le diacétate-éthidium bromure de fluorescinc (FDA-EB). Les populations viables ont été calculées, en prenant 3,58 x 10-15 g/bacilles solides comme moyenne du contenu en ATP d'une unité viable de Mycobacterium leprae. La proportion de bacilles viables a également été estimée dans les mêmes échantillons, en utilisant une méthode de coloration des bacilles solides (IM) et la recherche des bacilles colorés en vert par le FDA-EB. Chez les cas non traités, la viabilité mesurée par la méthode de l'ATP était de 100%; elle était de 92% par l'Index Morphologique, et de 100% par la méthode FDA-EB. Le contenu en ATP par bacille solide était relativement constant, ce qui n'était pas le cas pour les bacilles colorés en vert. Alors que l'Index Morphologique était 0 dans tous les cas, on pouvait encore déceler des bacilles viables par les estimations de contenu en ATP, chez 5 des 32 malades (16%) après 2 ans de polychimiothèrapie, et chez 1 sujet parmi 20 (5%) après 3 ans de PCT. Aucun bacille viable n'a pu être décelé môme avec cette méthode, après 3 années de traitement. Par ailleurs, des bacilles colorés en vert ont pu être démontrés chez 7 cas sur 32 (22%) après 2 ans de PCT, chez 2 cas sur 20 (10%) après 3 ans de PCT, et chez un cas sur 13 (8%) après plus de 3 ans de traitement. Ces résultats révèlent que la coloration par le FDAEB, de même que la méthode basée sur le contenu en ATP, ne permettent pas de mettre en évidence les mêmes populations de bacilles. La détermination du contenu en ATP de M. leprae pourrait être utilisée comme un outil fiable et sensible pour déterminer la viabilité des bacilles.RESUMEN
Se determinó la proporción de organismos viables en los bacilos purificados de 105 biopsias de 40 pacientes con lepra multibacilar sin tratamiento y de 65 pacientes multibacilares sujectos a terapia con múltiples drogas (TMD) por variados períodos de tiempo. Los bacilos se purificaron y su viabilidad se determinó en base a su contenido de ATP, al índice morfológico (1M), y a la tinción con diaectato de fluoresceína-bromuro de etidio (DAF-BE). Las poblaciones viables calcularon tomando 3.58 x 10-15 g como el contenido promedio de ATP de una unidad viable de Mycobacterium leprae. En los casos no tratados, la viabilidad fue del 100% usando el ensayo del ATP, del 92% según el IM, y del 100% con la tinción DAF-BE. El contenido en ATP por bacilo sólido fue relativamente constante pero éste no fue el caso en los bacilos con tinción verde fluorescente (DAF-BE). En situaciones donde el IM fue cero, cuantificando ATP todavía pudieron detectarse bacilos viables en 5 de 32 pacientes (16%) después de 2 años de TMD y en uno de 20 pacientes (5%) después de 3 años de TMD. No se encontraron bacilos viables después de 3 años de TMD. Por otro lado, se observaron bacilos con tinción verde fluorescente en 7/32 (22%) casos con 2 años de TMD, en 2/20 (10%) casos con 3 años de TMD, y en 1/13 (8%) con más de 3 años de tratamiento. Esto indica que la tinción con DAFBE y el ensayo de ATP no detectaron las mismas poblaciones. La cuantificación del contenido de ATP del M. leprae podría usarse como una medida confiable y sensible de la viabilidad del bacilo.The bacterial index (BI), a semiquantitative assessment of bacterial load, is commonly used for assessing response to therapy in multibacillary leprosy. However, the BI does not provide information about bacterial killing, and changes in the BI occur long after bacterial death. Since Mycobacterium leprae cannot be cultivated in vitro and since the mouse foot pad test takes a long time to yield results, there is a great need to develop rapid and reliable methods for monitoring the viability of M. leprae for assessing the efficacy of therapy. Several techniques have been developed for this purpose, but cither they have not been tried extensively or they have unacceptable limitations.
The percentage of solid-staining bacilli (morphological index; MI) has been used for nearly three decades to assess the effect of therapy (1,30). Kvach, et al. (14) reported the standardization of a fluorescent diacetate (FDA) and ethidium bromide (EB) staining procedure by which "living" bacteria are stained green. A significant decrease in the percentage of green-stained bacteria with increased periods of therapy in multibacillary leprosy has also been reported (14,22).
Adenosine-5-triphosphate (ATP) is present in all living cells in fairly constant amounts for each cell type. ATP is lost soon after cell death. These facts have provided the basis of the bioluminescent assay of biomass of several organisms (10,23,27). It has been reported that M. leprae synthesizes its own ATP (17). The ATP content of M. leprae has been measured by various workers (2,5,6,12,13,15,16), and a decrease in ATP levels after chemotherapy has been observed (4). Franzblau and Hastings (7) have described a drug screening assay based on ATP estimations from M. leprae incubated under in vitro conditions.
We have earlier reported on the standardization of purification, extraction, and assay procedures for the ATP measurement of M. leprae from clinical specimens (12,13). Preliminary results using this method showed that the ATP content generally correlated with solid populations as calculated by the MI but not with the counts of green bacilli using FDA-EB staining.
There is no published report of the comparative evaluation of live bacilli using the MI, FDA-EB staining, and ATP estimations in leprosy patients under multidrug therapy (MDT). We report here the results of such a comparison.
MATERIALS AND METHODS
Materials. Biopsies from 105 multibacillary [borderline lepromatous/lepromatous (BL/LL)] leprosy cases attending the outpatient department of our institute have been studied. Forty of these cases were untreated; 32 had received MDT for 2 years, and 20 for 3 years with a slightly modified WHO regimen (11); the remaining 13 had completed 38-45 months of MDT with the same regimen.
Methods. Coded biopsies were homogenized and decontaminated by a modified technique of Dhople and Storrs (6). Homogenization was done in 0.05 M phosphate buffer (pH 7.0), followed by decontamination with NaOH for 30 min at 37ºC. In the decontamination procedure, 2% NaOH (0.5 M) was finally used since this gave optimum results in our laboratory (12,13). After neutralization and centrifugation, the cells were treated with 0.1% w/v trypsin, chymotrypsin and collagenase; exposed to Triton X-100 (final concentration of 0.1% v/v); followed by ATPase treatment (final concentration 0.17% w/v, containing 0.005 M CaCI2). The final suspension was used for preparing two sets of smears on circular slides and also for the ATP assay. Slides were further coded and determinations of counts/g, MI, and FDA-EB staining were done in a blind manner.
One set of smears was stained with the Zichl-Neelsen stain, and total bacillary counts and morphological indices were calculated using the method of McRae and Shepard (21). The population of solid bacilli was estimated from the total counts of the MI.
The other set of smears was stained with fluorescein diacetate and ethidium bromide (FDA-EB) and examined under the fluorescent microscope by the method used by Kvach, et al. (M). From the total counts and percent of green-staining bacilli, the total green-staining bacillary populations were estimated.
ATP was extracted by the Tris-boiling method (13,29) . In our experience we have found this technique optimum and convenient for ATP extraction from cultivable mycobacteria and M. leprae (13).
ATP estimations were carried out in a Lumitran L-3000 ATP photometer (New Brunswick Scientific Co., New Brunswick, New Jersey, U.S.A.), using the modified method of Lchtokari, et al. (18). Modified assay conditions were: 200 μl of sample with proportionate changes in ATP monitoring reagents (LKB 1243-200) and Tris-acetate EDTA buffer; sensitivity of ATP photometer changed to 10 instead of conventional 7. One-minute integrated ATP counts were taken, and ATP concentration was calculated using the standard ATP from LKB (12,13). Using these altered conditions, it was found that the lower limit of detection for ATP, which produced a linear signal and was reproducible, was 0.05 pg (5 x 10-14 g). Three ATP estimations were done from each biopsy specimen and the mean values were taken.
After measuring the ATP content in the total bacillary population, ATP content per solid bacillus, and ATP content per green-staining bacillus were calculated (13).
For reasons explained in the Discussion, presuming the average ATP content of a solid bacillus (3.58 x 10-15 g) as the "viable unit," the most probable viable numbers in the bacillary suspensions were calculated.
RESULTS
MI, FDA-EB staining. Three of the 40 untreated cases had a zero MI. The morphological indices were 0.5% in 2, 1%-5% in 31, and 6%-9% in the remaining 4. The MI was zero in all the cases who had had treatment for 2 years or longer. The percentage of green-staining bacilli in the untreated cases ranged from 3.5%-15% in 11, 16%-30% in 21, and 31%-45% in the remaining 8. The figures for green-staining bacilli were 0-14%, 0-2%, 0-0.5% at the end of 2 years, 3 years, and 38-45 months of treatment, respectively. The present results confirmed our earlier observation (12,13) that there was no correlation between the viable populations estimated by the MI and green-staining bacilli (Tables 1 and 2).
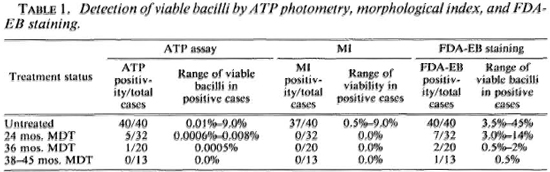
ATP content vs MI and FDA-EB staining. Among the biopsies from untreated cases, the ATP content per solid bacillus was fairly constant when the MI was 1% or more, and it ranged from 2.02 x 10-15 g to 5.60 x 10-15 g per solid bacillus (mean 3.58 x 10-15 g). In the same patients when the green-staining bacillus was presumed as the "viable bacillus," the ATP content per green-staining bacillus showed a wide variation. The ATP content per green-staining bacillus in these cases ranged from 0.22 x 10-15 g to 2.10 x 10-15 g among the different cases.
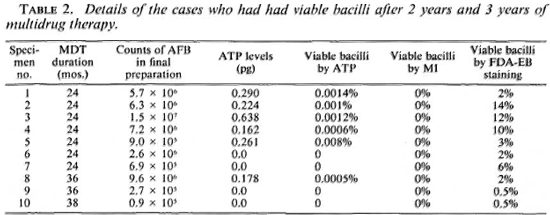
Detection of viable bacilli by MI, FDA-EB, and ATP in treated patients. The MI was zero in all of the 65 treated cases. Green-staining bacilli could be demonstrated in 10 of these 65 cases. In 6 of the 10 cases with green-staining bacilli, appreciable ATP levels in their bacillary suspensions were detected (Tables 1 and 2).
Estimation of viable populations by ATP. By ATP photometry, all 40 (100%) untreated patients had viable bacilli in their skin biopsies, and the proportion of viable bacilli ranged from 0.01% to 9.00% of the total bacillary population.
ATP estimates suggested that 27 of the 32 (84%) cases having 2 years of MDT had no viable bacilli. In the remaining five (16%) cases viable bacterial populations could be detected in their skin biopsies and their proportions ranged from 0.0006% to 0.0014% (4 cases) and 0.008% (1 case). After 3 years of MDT, 19/20 (95%) of the cases did not have detectable viable populations of ATP measurement; 1/20 (5%) had a demonstrable viable bacillary population. None of 13 cases who had completed 38-45 months of treatment were found to have any detectable ATP levels in the bacteria purified from their biopsies (Table 1).
DISCUSSION
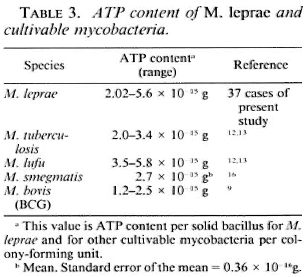
The ATP content of cultivable mycobacteria has been studied by various investigators (6,9,12,13,15,16,24). We have reported earlier that the ATP content of cultivable mycobacteria directly correlated with viable numbers and that it was a sensitive and stable index of viability (12-13). In these studies, it was also observed that when "solid staining" was taken as the indication of viability in untreated cases, the ATP content per viable bacillus was fairly constant. This ATP content was in the same range as found for a colony-forming unit (CFU) of other viable cultivable mycobacteria (Table 3) studied by us (12,13) and others (9,16). The results of the present study confirm our earlier observations. It is possible that some of the "damaged cells" may still be viable and some of the solids might have been "dead." Dormant bacilli may also have a different ATP content. This is also reflected in a larger range of ATP content/solid bacillus (viable cells) in M. leprae from different patients as compared to actively growing cells of cultivable mycobacteria (2.8-fold fluctuation in M. leprae as compared to 1.5- to 2.0-fold in cultivable mycobacteria (Table 3). But the contribution by these effects appears to be marginal. In any case, this limitation of mixtures of actively growing and dying (some still recoverable) organisms will be present even in the case of tube cultures with the determination of viability being done by CFU and other techniques. Thus, it appears reasonable to assume that solid-staining bacilli reflect the viable populations by and large. This lends further support to this concept postulated by earlier workers (20,21,25,26,31).
Since M. leprae cannot be cultivated on any known solid medium, it is not possible to determine the ATP content per CFU. Since the average ATP content per solid bacillus was found to be similar to that of a CFU of cultivable mycobacteria, we have made the reasonable assumption that this could be the content of a viable unit of M. leprae as well. By using the average ATP content per solid bacillus (3.58 x 10-15 g), we have estimated the most probable viable numbers in the purified bacillary suspensions from different cases. Our study demonstrates that, by ATP assay, viable bacilli are found in varying proportions in all untreated multibacillary cases with high Bis, and that there is a significant drop in viable populations in most of the treated cases. This conforms with Dhople's observations 4). ATP assay also shows that a significant proportion of patients continue to harbor viable bacilli even after 2 years of multidrug therapy (MDT). These cases were all negative by normal mouse foot pad testing as we have reported elsewhere (11). Our observations are similar to the results of the THELEP study in which the presence of viable bacilli has been reported in approximately 10% of the cases even after 24 months of MDT (28). It is apparent that the normal mouse foot pad technique is not sensitive enough to detect a very small proportion of viable bacilli in large dead populations (8,19). On the other hand, ATP estimations are based on total viable biomass and not the proportion of viable-to-dead organisms, as in the case of the normal mouse foot pad technique. The ATP assay method (12,13) thus appears to be a more sensitive tool for the detection of viable bacilli. The procedure in use at our laboratory appears to be as sensitive as those using immunocompromised animal models (28), besides being cheaper and faster to do. Because of therapeutic implications, biopsies should be repeated for ATP assay if the ATP content is very low and close to the detection limits of the particular assay system.
No data are available on the ATP content of metabolically active M. leprae and persisting bacilli, and we have assumed that they are similar. If, however, the persistcrs have a lower ATP content because of their possible diminished metabolic activity, the actual proportion of viable numbers would be greater than our estimates. It is thus possible that we could have underestimated the viable numbers in the treated cases. At present, there is no way to assess the error on this account.
Although a positive correlation between viability and solid-staining has been demonstrated in our studies and by others, the MI does not appear to be a reliable or sensitive index of viability for monitoring therapy, especially when zero values are recorded. In the present extended study, in 9 of the 68 biopsies with a zero MI appreciable ATP levels were detected, indicating that there were significant numbers of viable bacilli in these specimens. Further, mouse foot pad inoculation studies have also shown viable bacilli from cases with zero MIs (3). It is probable that due to the small sample and clumping of the bacilli, the solid bacilli are missed, resulting in a false zero MI reading (13).
It has been reported that the percentage of green-staining bacilli decreases with treatment (14,22). A similar trend was seen in the present study also (Table 1). Like the MI, FDA-EB staining may also have some role in monitoring the effect of therapy, but it appears that it may not be directly indicative of viability. If the green-staining bacilli were taken as the true index of viability, the ATP content per green-staining bacillus showed wide variations. The ATP content per green-staining bacillus varied up to tenfold in different cases. On the other hand, the ATP content per solid bacillus showed less than a threefold variation. The ATP levels were zero in four treated cases, yet significant percentages of green-staining bacilli were present (Tables 1 and 2). It appears that the enzymes responsible for the green staining in the FDA-EB technique take some time to decay after death; whereas ATP decays very fast and thus correlates better with viability. The morphological index and FDA-EB green-staining populations appear to detect different populations, as seen in the present study and as reported by Odinsen, et al. (22) and by us previously (12,13). Our study shows that the determination of the ATP content of M. leprae could be used as a more reliable and more sensitive tool for monitoring the effect of therapy than the less sensitive conventional morphological index.
Acknowledgments. The authors are thankful to Dr. H. Srinivasan, Director, for a constructive review of the manuscript. The technical assistance of Shri Surinder K. Bhan, Shri Ram, and A. Robi, and the secretarial assistance of Mr. J. D. Kushwah is gratefully acknowledged.
REFERENCES
1. DAVEY, T. F. Some recent chcmothcrapeutic work in leprosy. Trans. R. Soc. Trop. Med. Hyg. 54(1960)199-206.
2. DAVID, H. L., RASTOGI, N., FREHEL, C. and GHEORGHIU, M. Reduction of potassium tellurite and ATP content in Mycobacterium leprae. Ann. Microbiol. (Paris) 133B(1981)129-139.
3. DESIKAN, K. V. Correlation of morphology with viability of Mycobacterium leprae. Lepr. India 48(1976)391-397.
4. DHOPLE, A. M. Application of ATP assay to patient care in leprosy. In: XII International Leprosy Congress Proceedings, New Delhi, February 20-25 1984. Desikan, K. V., et. New Delhi: PRINTAID, n.d., pp. 358-360.
5. DHOPLE, A. M. and HANKS, J. H. Adenosine triphosphate content in Mycobacterium leprae, a brief communication. Int. J. Lepr. 49(1981)57-59.
6. DHOPLE, A. M. and STORRS, E. E. Adenosine triphosphate content of Mycobacterium leprae: effect of purification procedures. Int. J. Lepr. 50(1982)83-89.
7. FRANZBLAU, S. G and HASTINGS, R. C. Rapid in vitro metabolic screen for antileprosy compounds. Antimicrob. Agents Chemother. 31(1987)780-783.
8. GELBER, R. H., HUMPHRES, R. C. and FIELDSTEEL, A. II. Superiority of the neonatally thymectomized Lewis rat (NTLR) to monitor a clinical trial in Icpromatous leprosy of the two regimens of rifampin and dapsone. Int. J. Lepr. 54(1986)273-282.
9. GHEORGHIU, M. and LAGRANDERIE, M. Mcsure rapidede la viabilitcdu BCG per dosage de l'ATP. Ann. Microbiol. (Paris) 130B(1979)147-156.
10. HYSERT, D. W., KOVECSES, F. and MORRISON, N. M. Firefly bioluminescence ATP assay method for rapid detection and enumeration of brewery microorganisms. J. Am. Soc. Brew. Chem. 34(1976)145-150.
11. KATOCH, K., RAMU, G., RAMANATHAN, U. and SREEVATSA. Follow-up of BL/LL patients on a slightly modified WHO regimen of multidrug therapy. Indian J. Lepr. 59(1987)36-43.
12. KATOCH, V. M., KATOCH, K., RAMU, G., SHARMA, V. D., DATTA, A. K. and SHIVANNAVAR, C. T. Search for in vitro methods for determination of viability of mycobacteria: correlation of ATP content, morphological index and FDA-EB fluorescent staining in M. leprae. In: Proceedings of the Indo-UK Symposium on Leprosy, Agra, April 7- 10, 1986. Katoch, V. M., ed. Agra: Central JALMA Institute for Leprosy (ICMR), 1987, pp. 329-336.
13. KATOCH, V. M., KATOCH, K., RAMU, G., SHARMA, V. D., DATTA, A. K., SHIVANNAVAR, C. T. and DESIKAN, K. V. In vitro methods for determination of viability of mycobacteria: comparison of ATP content, morphological index and FDA-EB fluorescent staining in Mycobacterium leprae. Lepr. Rev. 59(1988)137-143.
14. KVACH, J. T., MUNGUIA, G. and STRAND, S. H. Staining tissue-derived Mycobacterium leprae with fluorescein diacctatc and ethidium bromide. Int. J. Lepr. 52(1984)176-182.
15. KVACH, J. T., NEUBERT, T. A., PALOMINO, J. C. and HEINE, H. S. Adenosine triphosphate content of Mycobacterium leprae isolated from armadillo tissue by percol buoyant density centifugation. Int. J. Lepr. 54(1986)1-10.
16. LEE, Y. N. and COLSTON, M. J. Measurements of ATP generation and decay in Mycobacterium leprae in vitro. J. Gen. Microbiol. 131(1985)3331-3337.
17. LEE, Y. N. and COLSTON, M. J. Adenylate kinase activity in Mycobacterium leprae. J. Gen. Microbiol. 132(1986)561-563.
18. LEHTOKARI, M., NIKKOLA, P. and PAATERO, J. Determination of ATP from compost using firefly bioluminescence technique. Eur. J. Microbiol. Biotechnol. 17(1983)187-190.
19. LEVY, L. Application of the mouse foot-pad technique in immunologically normal mice in support of clinical drug trials and a review of earlier clinical drug trials in lepromatous leprosy. Int. J. Lepr. 55 Suppl.(1987)823-829.
20. MCFADZEAN, J. A. and VALENTINE, R. C. The examination and the determination of the viability of Mycobacterium leprae by electron microscope. Lepr. Rev. 31(1960)6-11.
21. MCRAE, D. H. and SHEPARD, C. C. Relationship between the staining quality of Mycobacterium leprae and infectivity of mice. Infect. Immun. 3(1971)116-120.
22. ODINSEN, O., NILSON, T. and HUMBER, D. P. Viability of Mycobacterium leprae: a comparison of morphological index and fluorescent staining techniques in slit-skin smears and M. leprae suspensions. Int. J. Lepr. 54(1986)403-408.
23. POCCIOLO, G. L., CHAPPELLI, E. W., DENNING, J. W., MCGOORY, M. A., BIBLEY, D. A., OKREND, M. and THOMAS, R.R. Application of luminescent system to disease methodology. In: Technical Report No. X-726-76-212. Maryland: Goddard Space Flight Center (NASA), 1976.
24. PRIOLI, R. P., TANNA, A. and BROWN, I. N. Rapid methods for counting mycobacteria-comparison of methods for extraction of mycobacterial adenosine triphosphate (ATP) determined by firefly lucifcrasc assay. Tubercle 66(1985)99-108.
25. REES, R. J. W. and VALENTINE, R. C. The appearance of dead leprosy bacilli by light and electron microscopy. Int. J. Lepr. 30(1962)1-9.
26. SHEPARD, C. C. and MCRAE, D. H. Mycobacterium leprae in mice: animal infectious dose, relationship between staining quality and infectivity and cflcct of cortisone. J. Bacteriol. 89(1965)365-372.
27. SIRO, M. R., ROMAR, H. and GOVERN, T. Continuous flow method for extraction and bioluminescence assay of ATP in bakers' yeast. Eur. J. Appl. Microbiol. Biotcchnol. 15(1982)258-264.
28. SUBCOMMITTEE ON CLINICAL TRIALS OF THE SCIENTIFIC WORKING GROUP ON CHEMOTHERAPY OF LEPROSY (THELEP) OFTH E UNDP/WORLD BANK/ WHO SPECIAL PROGRAMME FOR RESEARCH AN D TRAINING TROPICAL DISEASES. The THELEP controlled clinical drug trials. Int. J. Lepr. 55 Suppl (1987)864-868.
29. THORE, A. Bioluminescence assay: extractions of ATP from biological specimens. Finland: LKB Wallac, 1979.
30. WATERS, M. F. R. and REES, R. J. W. Changes in the morphology of Mycobacterium leprae in patients under treatment. Int. J. Lepr. 30(1962)266-277.
31. WELCH, T. M., GELBER, R. H., MURRAY, L. P., NG, H., O'NEILL, S. M. and LEVY, L. Viability of Mycobacterium leprae after multiplication in mice. Infect. Immun. 30(1980)325-328.
1. M.D., Assistant Director; Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, U.P., India.
2. M.D., Senior Research Officer; Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, U.P., India.
3. M.B.B.S., D.P.M., Assistant Research Officer; Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, U.P., India.
4. M.Sc, Assistant Research Officer; Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, U.P., India.
5. M.Sc, Research Assistant; Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, U.P., India.
6. Ph.D., Assistant Research Officer; Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, U.P., India.
7. Ph.D., Deputy Director, Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, U.P., India.
Received for publication on 23 November 1988.
Accepted for publication in revised form on 13 February 1989.