- Volume 57 , Number 2
- Page: 492–8
Studies of human leprosy lesions in situ using suction-induced blisters 1. Cellular components of new, uncomplicated lesions
ABSTRACT
The cellular contents of blisters induced by suction over new, uncomplicated leprosy lesions, and in the skin of cured, control patients, have been examined with enzyme-and immuno-histochemical staining over a period of 4 days. The total cellularity of the blisters varied over a wide range, not correlated with the type of leprosy. Mononuclear cells predominated at all times studied, with nearly equal percentages of monocytes and T lymphocytes. The T-helper: suppressor ratio was significantly greater in BT than in BL and LL lesions at 48 hr. Suction blisters offer a painless, quantitative, reproducible, multiple-sampling method for obtaining cells f rom the cutaneous infiltrates of leprosy for phenotyping or functional analysis.RÉSUMÉ
On a examiné sur une période de 4 ans, au moyen de méthodes de coloration histochimiques des enzymes et immunohistochimiques, les contenus cellulaires de vésicules produites par succion sur des lésions neuves et non compliquées de lèpre, ainsi que dans la peau de malades témoins guéris. Le contenu cellulaire total des vésicules variait dans une large mesure, et n'était pas en corrélation avec le type de lèpre. Les cellules mononucléaires ont prédominé pendant toute la période d'étude, livrant des proportions à peu près égales de monocytes. Le rapport des lymphocytes T adjuvants et des suppresseurs était signilicativement plus élevé après 24 heures dans les lésions de lèpre tuberculoide dimorphe que dans celles de lèpre lépromateuse dimorphe ou lépromateuse. Les vésicules de succion constituent une méthode sans douleur, quantitative, reproductible, et pouvant être répétée dans de multiples échantillons, permettant d'obtenir des cellules à partir des infiltrais cutanés de lèpre, en vue de leur analyse phénotypique ou fonctionnelle.RESUMEN
Utilizando técnicas enzimo-e inmuno-histoquimicas se examinó, durante 4 días, el contenido celular de las vesículas inducidas por succión sobre lesiones dérmicas no complicadas de casos nuevos de lepra y sobre la piel de pacientes curados usados como control. La celularidad total de las vesículas varió en forma amplia y no correlacionó con ningún tipo de lepra en particular. En todos los tiempos estudiados predominaron las células mononueleares y los porcentajes de monocitos y linfocitos T fueron equivalentes. A las 48 horas, la relación de linfocitos T cooperadores: supresores fue significativamente mayor en los casos BT que en las lesiones BL y LL. Las vesículas inducidas por succión representan un método de muestreo múltiple, indoloro, cuantitativo, y reproducible, adecuado para obtener células de los infiltrados cutáneos de la lepra y para el análisis funcional y fenotípico de las mismas.Human immunity to Mycobacterium leprae is manifested by a wide spectrum of local inflammatory responses to M. leprae in the skin, producing the wide range of clinical and histologic changes observed in this disease (11,15). Most studies of the immunology of leprosy, however, have evaluated in vitro the functional status of cells obtained from the peripheral blood of patients, rather than examining cells from tissue lesions (1). The development of monoclonal antibodies against different lymphocyte membrane antigens has made it possible to examine the local infiltrate in situ in more detail (reviewed in 12). Such studies have shown that the percentages of phenotypically distinct lymphocyte sub-populations are different in the peripheral blood and the cutaneous lesions, and between lesions of different types of leprosy (9,16).
Although biopsies have the advantage of examining the site of immunologic activity and injury in situ, the trauma of the procedure does not readily permit the sequential, functional studies possible with peripheral blood cells in vitro. Moreover, if viable cells from biopsies are desired for functional studies, harsh physical or enzymatic methods are required to extract them from the connective tissue of the dermis.
We have attempted to combine the advantages of these two approaches by aspirating cells from blisters which have been induced by suction directly over skin lesions-a painless, nonscarring procedure (6,7). We here report the results of immunocytologic studies of the cells obtained from such blisters over representative skin lesions in 27 untreated patients across the immunopathologic spectrum of leprosy.
MATERIALS AND METHODS
Patients. Volunteers were recruited from newly diagnosed patients at McKean Rehabilitation Institute, Chiang Mai, Thailand. Patients with active leprosy were classified according to the clinical features of the lesions by two experienced physicians (TCS and VS) and histologic criteria in representative biopsies (DMS) according to a five-part scale (11). These included 11 lepromatous (LL), 9 borderline lepromatous (BL), 2 midborderline (BB), and 5 borderline-tuberculoid (BT) leprosy patients. Controls were recruited from cured patients with no evidence of reactivation, whose leprosy type was known from initial diagnostic examination by the same physicians in previous studies, confirmed in most cases by biopsy results (2,3). These included 9 former LL, 2 former BL, 1 former BB, 12 former BT, and 5 former tuberculoid (TT) patients.
Blister formation and cell preparation. Four blisters were induced painlessly by gentle continuous suction for 1-3 hr through a plastic template taped to the skin, as previously described (6). The template was placed directly over a representative skin lesion on the trunk or extremities in patients with active leprosy, or on the volar surface of the forearm in controls. Blisters were protected from injury by taping a small inverted cup over them.
Fluid was aspirated from blisters in a heparinized 1.0 cc "tuberculin" syringe in which the volume was measured. One blister was aspirated each day for 4 days; each blister was used only once.
The blister fluid was diluted to 1.0 cc with RPMI 1640 tissue culture medium, and the cells were collected on a 13 mm, 0.22 μm cellulose acetate filter (Millipore Corp., Bedford, Massachusetts, U.S.A.) (6). The filter preparations were fixed immediately in cold formol-acetone (14) for 30 sec, and washed in modified potassium phosphate-buffered saline (PBS) (17). While immersed in PBS, the filters were divided into several pieces, each of which was then stained with a different antibody or substrate.
Cell staining and counting. An indirect, two stage avidin-biotin method was used to stain individual pieces of each filter, using monoclonal antibodies Leu4, Leu3 or OKT4, and Leu2 or OKT8, to identify all T cells, T-helper and T-suppressor lymphocyte phenotypes, respectively. The secondary antibody was biotinylatcd sheep anti-mouse F(ab'), followed by an avidinbiotin-horseradish peroxidase complex (ABC). This substrate was developed using diaminobenzidine or aminoethyl carbazole (6). Monocytes/macrophages (MAC) were stained for nonspecific esterase (NSE) (18), and preparations were countcrstaincd with hematoxylin or methyl green. (Attempts to stain monocytes in this system with a variety of commercially available monoclonal antibodies did not produce sufficiently reproducible results, for reasons which are not yet clear.)
Cytologic detail was well preserved, easily permitting discrimination between mononuclear cells and polymorphs. Similarly, the different patterns of NSE staining between lymphocytes and monocytes were easily discerned.
The percentage of positively stained cells (i.e., each subset) was determined by a differential count of at least 200 cells in each segment of the filter; the mean number of cells in 20 oil immersion fields was used to calculate the total number of cells on the filter, finally expressed as the number of cells per mm 3 of blister fluid.
From each filter, one control segment was incubated with secondary antibody followed by the ABC reagent, and another with ABC reagent only, prior to development with substrate and indicator. In addition, control preparations of peripheral blood leukocytes, collected on filters and washed and fixed in identical fashion, were stained with every blister preparation. Background staining (for both blister and peripheral blood preparations) was usually slight, and presented no problem in interpretation. If controls (or blister samples) showed evidence of high background, low or irregular positive staining, or were otherwise technically unsatisfactory for any antibody, the data for that antibody were not included in the analysis.
Statistical analysis. Quantities and ratios were ranked and tested using the nonparametric Wilcoxon two-sample test.
RESULTS
The volume of fluid obtained from blisters at all times after induction ranged from 30-150 μl (median = 60; mean = 66); the range and distribution of volume did not differ significantly between the active patients and the controls. In blisters induced in the skin of cured, control volunteers the median total cellularity varied from 80-400 cells/mm3 (ranging from 50-550 cells/mm3) over all times studied (Fig. 1). Cellularity was higher during the first 24 hr, as part of the nonspecific inflammatory response to blister induction (6). No statistically significant differences were seen among patients who had had different types of leprosy earlier.
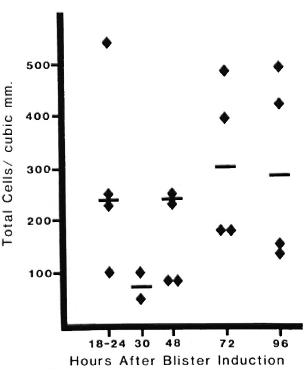
Fig. 1. Cellularity of blisters in cured, inactive control patients. Four blisters were induced (as described in text) on the volar surface of the forearm in patients with no evidence of active disease. Blisters were aspirated at different intervals after induction. Each point = cell count from one blister; bar = median value.
To determine the reproducibility of cell sampling using the blister technique, two blisters were aspirated at the same time from a number of control patients. The paired results gave a correlation coefficient of 0.613 (p = 0.003) (Fig. 2).
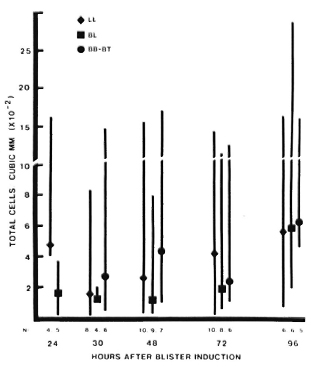
Fig. 2. Correlation of cell counts in paired blisters. Four blisters induced on the forearm of cured, inactive patients were sampled in pairs at indicated intervals after blister induction. Total cell counts were determined independently for each blister. Correlation coefficient for these paired samples is 0.613; slope of the curve was determined by linear regression.
In active leprosy lesions, total cell counts varied much more widely than in the controls, ranging from 1-2800 cells/mm3 (Fig. 3). Median values for the different immunopathologic types of leprosy were similar at all times studied.
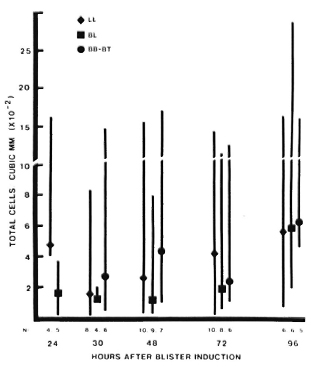
Fig. 3. Total cell counts in blisters over active, uncomplicated leprosy lesions. Four blisters were induced directly on active lesions which were classified by clinical and histologic criteria. Blisters were aspirated only once, at different intervals after induction. For each type of lesion: point = median value; vertical bar = range; N = number of observations.
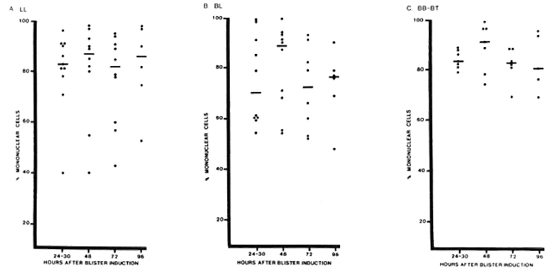
Mononuclear cells predominated in active lesions, with median percentages of 70%-91% over all times studied (Fig. 4). Polymorphs accounted for more than 30% of the cells in blisters over some BL lesions (3 patients) and LL lesions (2 patients), but not in BB or BT lesions. Neither eosinophils nor basophils were present in substantial numbers.
Fig. 4. Percentage of mononuclear cells in blisters over different types of leprosy lesions. Percentage of mononuclear cells (lymphocytes and monocytes) was determined for blisters aspirated at different times after induction on different types of leprosy lesions. Each point = one blister; horizontal bars = median values.
Among mononuclear cells, the median ratio of T cells: monocytes (Leu4+ : NSE+ cells) remained between 0.5-1.5 for all types of leprosy at all times studied, except for a predominance of T cells at 48 hr in BT lesions (Fig. 5). This difference was statistically significant (p < 0.005), and it is notable that this was observed in blisters with similar total cell counts (Fig. 3), indicating an absolute as well as a relative increase in T cells in BT lesions.
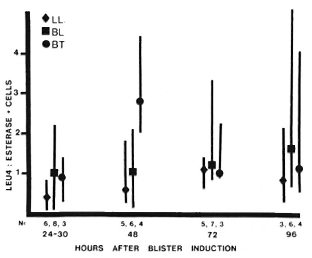
Fig. 5. T-cell monocyte ratios in leprosy lesions. Among cells obtained from blisters at different times after induction over leprosy lesions, total T-cell numbers were determined using anti-Leu4 antibody; monocytes were identified by staining for nonspecific esterase. For each type of lesion: point = median value; vertical bar = range: N = number of observations. Difference between BT and BL-LL 48 hr after induction is statistically significant (p < 0.005).
At 48 hr, the median helper: suppressor (H:S) (CD4:CD8) ratio was 3.3 in BT lesions and 1.3 in BL and LL lesions, a statistically significant difference (p < 0.005). The median H:S ratio ranged from 1.0-3.0 at other times, but the range of values was also great and at none of these times were the differences statistically significant (Fig. 6).
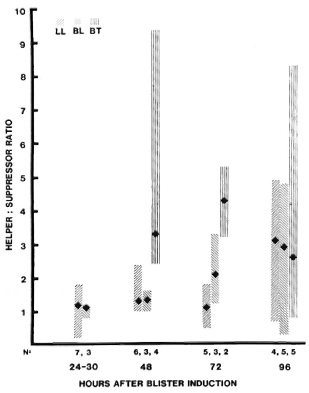
Fig. 6. T-helper: suppressor ratios in uncomplicated leprosy lesions. Among cells obtained from blisters at different times after induction over leprosy lesions, T-helper and -suppressor phenotypes were determined by immunostaining with anti-Leu3 and OKT8 antibodies, respectively. For each type of lesion: point = median value; vertical bar = range; N = number of observations. Difference between LVT and liL-LL 48 hr after induction is statistically significant (p < 0.005).
DISCUSSION
This study addresses the feasibility of using suction-induced blisters to evaluate the relative numbers and types of cells in the cutaneous infiltrates of leprosy, with minimal trauma to the patient or the cells. Rebuck's "skin windows" (10) were the conceptual forerunner of this approach. Other investigators attempted to apply Rebuck's technique to leprosy (4 5), but the cutaneous abrasion required is difficult to standardize, and meaningful data concerning mononuclear cells was obscured by the brisk polymorphonuclear responses induced by cover slips. As noted by the originators of the blister technique (8), this method introduces no foreign material of any kind and therefore approximates more closely the conditions of the intact skin. The suction used to induce the blisters does elicit a mild, nonspecific inflammatory response which peaks during the first 24 hr in healthy volunteers 6). In this study, samples were obtained from 24 hr onward to minimize the effects of this nonspecific response.
The total cellularity of the blisters over active lesions varies greatly, not unlike the variations in cellularity observed in the lesions in histologic sections (11). The cellularity of blisters itself is therefore not a valuable indicator of the immunologic status of the underlying lesion, although it is also notable that many blisters over active lesions had many more cells than the highest numbers seen in blisters in cured, inactive patients. The marked kinetic changes which may be seen with this technique during an accelerating immune response (6) were neither expected nor observed in these lesions, since no antigenic stimulation was used to induce an immune response in this study. The result is, instead, a profile of the "baseline" status of different types of leprosy lesions.
The method shows an acceptable degree of reproducibility from blister to blister, as evidenced by the data on paired blister samples. Some of the scatter which is seen is probably due to imperceptible differences in trauma during blister induction, or to variations in the quantity of red blood cells, which is usually negligible but was not quantitatcd in this study.
The high percentage of mononuclear cells within the blisters is in agreement with the histologic features of these lesions (11), and indicates that the method is suitable for the study of the cells involved in immunologic responses in leprosy. The percentage of polymorphs infrequently exceeded 25%30% of the total and was, in most instances, far less. Other investigators have demonstrated that the introduction of very few bacteria is sufficient to induce an acute inflammatory response in a blister (8), and we view the occasional occurrence of numerous polymorphs in one blister (out of a set of 3-4 in a volunteer) as likely to be the result of such contamination due to a crack or puncture of the blister. Interestingly, however, among patients in whom 3-4/4 blisters contained over 30% neutrophils, the majority developed a reaction (cither type 1 or 2) soon thereafter. The extent and significance of this observation are under investigation.
Monocytes/macrophages were a major cellular component in blisters over active lesions, consistent with their prominent place in all types of leprosy lesions. However, monocytes/macrophages were also numerous in blisters in cured, control patients, as they were in earlier studies of healthy volunteers (6 and Scollard, unpublished results). The activation status and functional activity of the monocytes/macrophages may differ in these different situations, but these parameters were not assessed in this study.
In BT lesions, T-helper/inducer phenotypes outnumbered T-suppressor/cytotoxic phenotypes at least 3 to 1 at 48 hr after blister induction, in agreement with the patterns observed in biopsy studies (9,16). Recruitment of T cells into the blister appeared to continue after this time, probably nonspecifically, and the trend was no longer evident at 96 hr. The statistically significant results at 48 hr, however, indicate that the method does provide a sample of the cutaneous infiltrate, reflecting the status of the lesion.
Phenotype alone does not indicate the functional status of the cells. Preliminary studies suggest that direct assessment of some functional capabilities of blister exudate cells is possible, however, and further work is in progress.
The blister technique has proved to be very acceptable to patients, enabling multiple sequential studies of the same patient. Another distinct advantage of this method over biopsies is the ability to make more precise determinations of the total number of cells present in the blister-an accurate denominator for quantitative studies of cell subsets. The precise origin of every cell in the blister is not known, and some cells may be recruited into the blister from dermal capillaries rather than from the immunoinflammatory infiltrate. Nevertheless, previous studies with purified protein derivative (PPD) skin tests have demonstrated that the blister does reflect the immunologic activity in the dermis, as indicated by correlations between 48 hr induration and increased numbers of cells (6) and of soluble interleukin-2 (IL-2) receptor (Tac peptide) (13) in blisters induced over the PPD reaction. The differences in the H:S ratio in blisters over lepromatous versus tuberculoid lesions to a level similar to that seen in biopsies suggest that this is also the case with leprosy lesions. Together, these results indicate that suction-induced blisters offer a quantitative reproducible method, minimally traumatic and amenable to multiple sampling, with which cells can be obtained which are representative of the cutaneous infiltrates in leprosy lesions.
Acknowledgments. This work was supported by PSTC Grant No. 5.141 from USAID, and by a Research Strengthening Grant from the WHO/TDR program to the Research Institute for Health Sciences, Chiang Mai University. Chiang Mai. Thailand.
We are most grateful to Atcha Masarat. Utaiwan Utaipat. and Rawiwan Kurapakorn for their excellent nursing and technical assistance.
REFERENCES
1. BLOOM, B. R. Learning from leprosy: a perspective on immunology and the Third World. J. Immunol. 137(1986)i-ix.
2. BROWN, A. E., NELSON, K. E., MAKONKAWKEYOON, S., VITHAYASAI, V., SCOLLARD, D. M. and BULLOCK, W. E. A study of the immunological effects of cimetidine in patients with lepromatous leprosy. Int. J. Lepr. 53(1985)559-564.
3. BROWN, A. E., VITHAYASAI. V., SCOLLARD, D. M., NELSON, K. E., MOSES, V., SCHAUF, V. and MAKONKAWKEYOON. S. Lymphocyte transformation in lepromatous leprosy: a study of the influence of disease activity and symptom duration. Southeast Asian J. Trop. Med. Public Health 17(1986)104-110.
4. BULLOCK, W. E., HO, M. F. and CHEN, M. J. Quantitative and qualitative studies of the local cellular exudative response in leprosy. J. Reticuloendothel. Soc. 16(1974)259-268.
5. CARRIE, S. A. and LEVAN, N. Cell-window tests in lepromatous leprosy. (Letter) Lancet 1(1970)1116.
6. KENNEY, R. T., RANGDAENO, S. and SCOLLARD, D. M. Skin blister immunocytology: a new method to quantify cellular kinetics in vivo. J. Immunol. Methods 97(1987)101-110.
7. KIISTALA, U. and MUSTAKALLIO, K. K. In-vivo separation of epidermis by production of suction blisters. Lancet 1(1964)1444-1445.
8. Kiistala. U., Mustakallio, K. K. and RORSMAN, H. Suction blisters in the study of cellular dynamics of inflammation. Acta Derm. Venereol. (Stockh.) 47(1967)150-153.
9. MODLIN, R. L., HOFMAN, F. M., TAYLOR, C. R. and REA. T. H. T lymphocyte subsets in the skin lesions of patients with leprosy. J. Am. Acad. Dermatol. 8(1983)182-189.
10. REDUCK, J. W. and CROWLEY, J. H. A method of studying leukocyte functions in vivo. Ann. N.Y. Acad. Sci. 59(1955)757-805.
11. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
12. SCOLLARD, D. M. A review of immunopathologic studies of human leprosy lesions in situ. Hawaii Med. J. 47(1988)54-59.
13. SCOLLARD, D. M., WAGNER, D. K. and NELSON, D. L. Release of soluble interleukin-2 receptors (Tac peptide) in vivo during human immune responses to tuberculin. Clin. Immunol. Inimunopathol. 46(1988)450-455.
14. SCHWINN, C. P. and FERGUSON, C. T. Cytopreparatory techniques. In: Compendium of Diagnostic Cytology. Wied, G. L., Koss, L. G. and Reagan, J. W., eds. Chicago: Tutorials of Cytology, International Academy of Cytology, 1976, 4th ed., pp. 360-369.
15. SKINSNES, O. K. The immunological spectrum of leprosy. In: Leprosy in Theory and Practice. Cochrane, R. G. and Davey, T. F., eds. Baltimore: Williams and Wilkins, 1964. pp. 156-182.
16. VAN VOORHIS. W. C, KAPLAN, G., SARNO. E. N., HORWITZ, M. A., STEINMAN, R. M., L EVIS, W. R., NOGUEIRA, N., HAIR, L. S., GATTASS, C, ARRICK, B. A. and COIIN, Z. A. The cutaneous infiltrates of leprosy: cellular characteristics and the predominant T-cell phenotypes. N. Engl. J. Med. 307(1982)1593-1597.
17. WOOD, G. S. and WARNKE, R. Suppression of endogenous avidin-binding activity and its relevance to biotin-avidin detection systems. J. Histochem. Cytochem. 29(1981)1196-1204.
18. YAM, L. J., Li, C. Y. and CROSBY, W. H. Cytochemical identification of monocytes and granulocytes. Am. J. Clin. Pathol. 55(1971)283-290.
1. M.D.; Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
2. M.D.; Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
3. M.D., Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
4. M.D., Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
5. M.D., Ph.D., Department of Pathology, John A. Burns School of Medicine, University of Hawaii, Honolulu, Hawaii 96816, U.S.A.
6. M.B.B.S., McKean Rehabilitation Institute, Chiang Mai, Thailand.
Reprint requests to Dr. Scollard.
Received for publication on 17 October 1988.
Accepted for publication in revised form on 11 January 1989.