- Volume 64 , Number 1
- Page: 58–65
Development of a SOD ELISA to determine the immunological relatedness among mycobacteria
ABSTRACT
This study reports on the standardization of an enzyme-linked immunosorbent assay (ELISA) system for the measurement of immunological distances (ImDs) of the superoxide dismutase (SOD) molecule among the cultivable mycobacteria, namely, Mycobacterium vaccae, M. phlei, M. smegmatis, M. avium, M. scrofulaceum, M. tuberculosis H37Ra, M. tuberculosis H37Rv, and M. bovis BCG, and M. leprae. SODs f rom cultivable mycobacteria were purified, antibodies were raised against these molecules, and ImDs between these anti-SOD antibodies and antigen (SODs) were determined by an immunoprecipitation technique standardized earlier and by the ELISA technique developed in this study. The ELISA system developed in this study showed higher sensitivity and consistent and reproducible ImDs among various mycobacteria, including pathogens such as M. tuberculosis, M. leprae and M. avium. These values were comparable with the values derived by the immunoprecipitation technique. Our ELISA technique appears to be a sensitive and rapidly reproducible method with the additional advantage of the stability of reagents, and holds promise in the taxonomy as well as in the development of diagnostics for leprosy and other mycobacterial infections.RÉSUMÉ
Cette étude concerne la standardisation d'un système enzymatique (ELISA) pour la mesure des distances immunologiques de la molécule de dismutase superoxide (DOS) parmi les mycobactéries cultivables Mycobacterium vaccae, M. phlei, M. smegmatis, M. avium, M. scrofulaceum, M. tuberculosis H37Ra, M. tuberculosis H37Rv, et le BCG à M. bovis) et M. leprae. Les DOS des mycobactéries cultivables ont été purifiées, on a provoqué la production d'anticorps contre ces molécules, et les distances immunologiques entre ces anticorps anti-DOS et l'antigène ont été déterminées par une technique d'immunoprécipitation standardisée précédemment, et par la technique ELISA développée dans cette étude. Le système ELISA développé dans cette étude a montré une sensibilité plus élevée et des distances immunologiques cohérentes et reproductibles parmi les différentes mycobactéries, y compris les pathogènes tels que M. tuberculosis, M. leprae et M. avium. Ces valeurs étaient comparables aux valeurs dérivées par la technique d'immunoprécipitation. Notre technique ELISA apparaît être une méthode sensible et rapidement reproductible, avec l'avantage supplémentaire de la stabilité des réactifs, et elle est pleine de promesses pour la taxonomie aussi bien que pour le développement de méthodes diagnostiques pour la lèpre et d'autres infections mycobactériennes.RESUMEN
Este estudio se refiere a la estandarización de un ensayo inmunoenzimático (ELISA) par a la medición de las distancias inmunológicas (ImDs) de la molécula de superóxido dismutasa (SOD) entre Mycobacterium leprae y las micobacterias cultivables M. vaccae, M. phlei, M. smegmatis, M. avium, M. scrofulaceum, M. tuberculosis H37Rv, y M. bovis, BCG. Las SODs de las micobacterias cultivables fueron purificadas, contra ellas se prepararon anticuerpos, y las lmDs entre estos anticuerpos anti-SOD y los antígenos (SODs) se determinaron por una técnica de inmunoprecipitación estandarizada antes y por la técnica de ELISA desarrollada en este estudio. El sistema de ELISA aquí desarrollado mostró mayor sensibilidad y dio ImDs consistentes y reproducibles entre varias micobacterias, incluyendo a patógenos tales como M. tuberculosis, M. Ieprae y M. avium. Estos resultados fueron comparables con los valores derivados de la técnica de inmunoprecipitación. Nuestra técnica de ELISA parece ser un método sensible, rápido y reproduciblc, que tiene la ventaja adicional de la estabilidad de los reactivos. El método promete ser de utilidad en estudios taxonómicos y en el desarrollo de pruebas de diagnóstico de la lepra y de otras enfermedades micobacterianas.Serological techniques can be used to identify and to characterize pathogenic organisms, including mycobacteria, directly from clinical specimens even without in vitro isolation of the organisms (14). Such tests mainly depend on the reaction of single or multiple antigenic components of bacteria with antibodies against them. Depending upon the amino-acid sequence and structural configuration, the antigenicity of proteins (bacterial components) differs from species to species. These changes in the amino-acid sequences of enzymes (protein) are measurable by immunological crossreactivity and have been observed to reflect evolutionary relatedness or divergence (2,3,26).
A number of indirect immunological techniques/assays have been developed to measure the difference in the amino-acid sequences of the polypeptide chain of a single protein/enzyme. Among these methods, complement fixation, precipitation, and immunodiffusion techniques have been used to study the phylogenetic and evolutionary divergences among various species of different genera (3,7,9,21,30). In mycobacteria, catalases have been analyzed for the measurement of the immunological distances (ImDs) by using techniques such as macroor micro-immunoprecipitation (14,15,31,32). While these techniques have various technical limitations on the stability of enzymatic activity and the quantities required, the major problem for Mycobacterium leprae was the absence of any demonstrable immunoreactive catalasc in the cell-free extracts of M. leprae derived from armadillos (15). To overcome these dillicultics, another widely distributed molecule, superoxide dismutase (SOD) (11,17,18,33), was chosen and preliminary results showed its potential (13.28).
This communication describes the development of an ELISA-based system to determine the immunological relatedness among different mycobacterial SODs and also to compare the trends discerned by this technique and conventional immunoprecipitation methods.
MATERIALS AND METHODS
Mycobacterial strains. Mycobacteria included in this study were M. vaccae (Stanford 877R), M. phlei (NCTC 8156), M.smegmatis (TMC 1546), M. tuberculosis H37Ra (NCTC strain), M. tuberculosis H37Rv (TMC 102), M. bovis BCG (Glaxo), M. avium (TMC 706), M. scrofulaceum (TMC 1302) and M. leprae (armadillo-derived). The cultivable mycobacterial strains included in this study were initially obtained from Dr. J. Stanford, London; National Institutes of Health, Bethcsda, Maryland, U.S.A., and Central Drug Research Institute, Lucknow, India, and are being maintained in the Microbiology Laboratory at our Institute. M. leprae was obtained from the livers and spleens of armadillos infected at this Institute with human leproma and stored at - 70ºC.
Growth and medium. Initially, all the cultivable mycobacterial strains were grown on Lowenstein-Jensen (LJ) medium (22), then subcultured into the liquid Sauton's medium (27), and incubated at 37ºC until luxurient growth was obtained. The logphase growths were collected by centrifugation and washed with 0.01 M phosphate buffer (pH 7.2) and stored at -20ºC until used. M. leprae were purified from the infected armadillo liver and spleen by the percoll density gradient centrifugation-biphasic separation method of Draper (6) and were checked for any cultivable mycobacteria.
Preparation of cell-free extract (CFE) and purification of SOD enzyme. The CFEs from cultivable mycobacterial species were prepared by passing the growth suspended in phosphate buffer (1:5) through the prechilled French pressure cell press at 28,000 psi (AMINCO, SLM Instruments, Inc., Urbana, Illinois, U.S.A.) or by sonication (Lucas Dawe Ultrasonics, U.K.) (10). Clear CFEs were obtained by centrifugation at 28,000 x g for 30 min at 4ºC. A part (10 ml) of the CFE from each strain was stored at - 70ºC until used; the rest of the extract was used for purification of SOD enzyme by the modified methods (16,17). Briefly, KC1 was added to the CFE to a final concentration of 0.1 M, heated for 5 min at 65ºC, and cooled to below 10ºC immediately in an ice bath. The nucleic acids and ribosomes were removed from the KC1-treated supernatant by treating with streptomycin sulfate (2.5% w/v final concentration). The clear supernatant from the above step was made to 65% saturation with solid ammonium sulfate. After removing the precipitate, the supernatant was again made to 85% saturation with ammonium sulfate and incubated at 4ºC overnight. The precipitate was saved and dissolved in a minimum volume of phosphate buffer. This was dialyzcd overnight against the 0.1 M phosphate buffer of pH 7.0 at 4ºC. The dialyzed solution was used for the final purification of SOD by 7.5% polyacrylamide gel electrophoresis (PAGE). The CFE from M. leprae was prepared by the same method using a mini French pressure cell press.
Purification of SOD enzyme by polyacrylamide gel electrophoresis (PAGE). The partially purified SOD enzyme preparations fractionated with 85% saturation of ammonium sulfate were subjected to 7.5% PAGE (4), and the purification of the SOD enzyme was repeated until a single protein band coinciding with the achromatic enzyme activity band was obtained on 10% PAGE. The purified SOD enzyme was further checked for purity by SDS-PAGE.
PAGE staining procedures. The polyacrylamide gels were stained after electrophoresis for SOD enzyme activity by the method of Beauchamp and Fridovich (1), and proteins were stained by the method of Holbrook and Leaver (8).
Measurement of SOD activity. SOD enzyme activity in CFE preparations obtained in different steps of enzyme purification was estimated spectrophotometrically by the technique of Nishikimi, el al. (25) and Kakkar, el al. (12).
Raising of antibodies against purified SOD. Each purified mycobacterial SOD enzyme was mixed and emulsified with 1 mg of immuno-adjuvant peptide ( N -acetylmuramyl-L-alanyl-D-isoglutamine-MDP, Sigma) and incomplete Freund's adjuvant (IFA; DIFCO/Sigma) as per the method of Wayne and Diaz (31). One ml of this emulsified mixture was injected intradermally/ subcutaneously into the New Zealand white rabbit at 5-8 sites. These same injections were repeated on day 21 and day 40. Finally, the booster (SOD enzyme intravenously) was given on day 54, and the rabbits were bled 7 days later. The serum was separated and stored at -20ºC in aliquots until used.
Purification of immunoglobulins. The hyperimmune scrum was precipitated with an equal volume of saturated ammonium sulfate solution (31), and the precipitation was repeated twice. The precipitate mixture was allowed to stand for 2 hr and was then centrifuged. The pellet was dissolved in a minimal volume of phosphate buffer of pH 7.2 and was then dialyzed against 0.1 M phosphate buffer of pH 7.2 at 4ºC. Finally, the volume was made equal to the original volume of scrum taken with phosphate buffer.
Serological assays. Immunoprecipitation of SOD with homologous and heterologous antibodies was carried out with a slight modification of the method originally developed and used for estimation oflmDs between two mycobacterial species using catalase enzyme as a marker by Wayne and Diaz (31). The assay procedure used in this study was as follows: one unit of SOD solution was added to equal volumes of serially diluted homologous and heterologous antibodies in 1.5-ml centrifugation tubes, mixed well, incubated at 37ºC for 1 hr, followed by incubation at 4ºC overnight. The supernatant was separated and used to measure the unbound SOD present by the spectrophotometcric method described above. Rabbit sera collected before injection were used as controls.
ELISA. Based on the principles of analogous systems (24,34), an ELISA for measuring the binding of SOD and anti-SOD antibodies was developed. This was used for estimating the binding of mycobacterial SOD with homologous and heterologous anti-SOD antibodies. The essential steps of this procedure are as follows: 1) The purified SOD enzyme, 25 µ g/ml (antigen) was dissolved in carbonate bicarbonate buffer, pH 9.6 (coating buffer). 2) Each well, except the last two wells, of a microliter plate (Cooke Microtitcr, U.S.A.) received 100 µ l of a coating buffer containing SOD (25 µ g/ml). The last two wells received only the coating buffer, and the plates were incubated overnight at 4ºC. 3) Wells were washed three times with phosphate bufler saline with Tween-20 (PBS-T), 100 µ l of bovine serum albumin fraction-V (BSA-V; 2 mg/ml) in PBS-T was added to each well, and the plates were incubated at 37ºC for 1 hr. 4) After washing the wells, 100 µ l of serially diluted test and control rabbit sera (immunoglobulins) was added to respective rows and columns except for the number 1 well (antigen control) and the last well (substrate control). The second to last well of the microtitcr plate received undiluted homologous serum (immunoglobulins). The plates were incubated at 37ºC for 3 hr in a humid chamber. 5) The plates were washed with PBS-T three times, and the traces of buffer in the wells were removed. Then, 100 µ l of diluted (1: 5000) anti-rabbit immunoglobulins conjugated with horseradish peroxidase in the PBS-T (Dakopatts) was added. The plates were shaken carefully and incubated overnight at 4ºC in a humid chamber. 6) The unbound enzyme-labeled antibodies were washed with PBS-T four times. 7) 100 µ l of 0.1% o -phenylene diamine (OPD) in citric acetate buffer (pH 5.0) containing 0.03% H2O2 (to be prepared just before use) was added and incubated at room temperature for 30 min in the dark. 8) The reaction was stopped by adding 25 µ l of 8 NH2SO4 to each well. 9) The color developed was measured at 490 nm using an ELISA reader (Microliter reader; Model 700: Dynatech Labs, Inc., Chantilly, Virginia, U.S.A.) and the titer of each test serum was calculated. The first well without test antibody was taken as blank, the antigen control (second to last well) was an antibody control, and the last well was a substrate control.
Calculation of immunological distances (ImDs). The binding capacity of serum against a given SOD preparation was the reciprocal of that dilution of serum which precipitated (in immunoprecipitation technique) or bound (in ELISA) 1 unit or a delined amount of SOD. These were calculated from the points on the titration curve closest to the 50% endpoint on the probability graph paper, as done in the catalasc system earlier (31).
RESULTS
The ELISA system standardized in this study elicited consistent and reproducible immunological distances between the homologous and the heterologous mycobacterial SODs. Antigen-coated plates were stable and shared consistent results up to 25 µ g/ml (SOD enzyme).
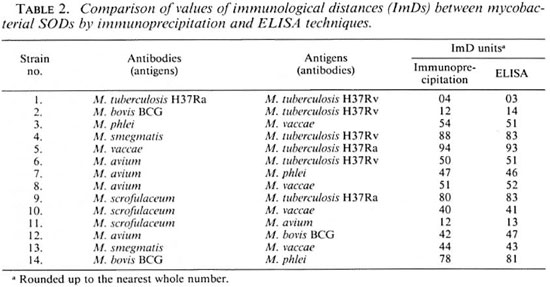
Calculation of ImDs using OD serological titers by ELISA. The binding titers of serially diluted anti-sera (1:16, 1:32, 1:64, 1:128, 1:256, 1:512, ... 1:8192) raised against SODs of cultivable mycobacteria were determined with homologous SODs (25 µ g/ml) by the ELISA technique. The ImD units calculated between the homologous and heterologous antigen-antibody titrations of eight cultivable mycobacteria are shown in Table 1. The reciprocal ImDs of M. tuberculosis H37Ra anti-SOD antibodies versus M. bovis BCG SOD and M. bovis BCG (serum) versus M. tuberculosis H37Ra (SOD) were found to be 11 and 12 ImD units, respectively, by the two methods. The ImDs calculated for other species were found to be similar, consistent, and reproducible by both immunoprecipitation and ELISA methods. Table 2 compares some of the ImDs estimated between the SODs of different cultivable mycobacteria by immunoprecipitation and ELISA techniques. It should be noted that very similar ImDs were discerned by these two techniques.
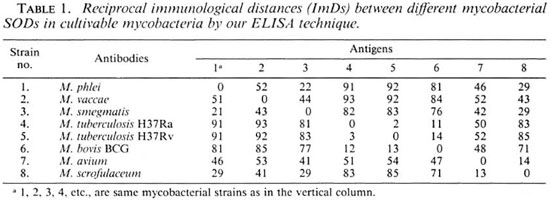
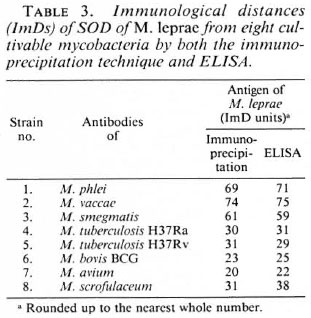
The ImD values between M. leprae and other cultivable mycobacteria included in this study, as calculated by the titration of anti-SOD antibodies raised against SODs from cultivable mycobacteria versus SOD antigen from M. leprae by ELISA and immunoprecipitation techniques, are shown in Table 3. ImD units calculated from titrations by the immunoprecipitation (P) and ELISA (E) techniques were similar for M. leprae versus all of these eight species. The ImDs of SOD from M. leprae to various species were: M. phlei (69P vs 71E), M. vaccae (74P vs 75E), M. smegmatis (61P vs 59E), M. tuberculosis H37Ra (30P vs 31E), M. tuberculosis H37Rv (31P vs 29E), M. bovis BCG (23P vs 25E), M. avium (20P vs 22E) and M. scrofulaceum (31P vs 38E), respectively.
DISCUSSION
According to Cocks and Wilson (2), the immunological distance (ImD) is a logarithmic function of the index of dissimilarity between two biological systems, and is directly related to the differences in the amino-acid sequence of a particular protein. These ImDs between various biological systems have been estimated and have been reported to correlate with the structural divergence of protein, up to substitution of about 40% amino-acid sequences (3,5,26). The immunoprecipitation, immunodiffusion, sero-agglutination and microcompliment fixation tests and the ELISA are some of the techniques used for this purpose in various biological systems, including mycobacteria (2,14,15,26,31). Except for the ELISA, most of these techniques require larger quantities of antigen and antibodies, and the sensitivity of the assays arc generally low.
To determine the immunological distances of mycobacterial catalases, Wayne and Diaz (31,32) used the immunoprecipitation technique. Because of a lack of the presence of an adequate quantity of immunoreactive catalases in the cell-free extracts (CFEs) in M. leprae (15), another widely present enzyme, superoxide dismutase (SOD), has been chosen as a target molecule for the measurement of ImDs and has shown promising results (13). This enzyme has been shown to be present in various mycobacteria (11,13,17,18,33) and has been demon strated to possess a qualitative difference in mycobacteria (23) and other organisms, such as those belonging to Acholeplasmataceae (20). We have earlier standardized an immunoprecipitation technique (13) to measure these divergences in the SOD molecule, and observed that there could be a problem in estimating ImD values by the immunoprecipitation technique at different intervals, mainly due to the loss of SOD activity on long-term storage in the laboratory. Degraded or denatured enzyme antigen resulted in using variable concentrations of the antigen in the assay which led to discordance since this assay is based on the estimation of unbound enzyme in the supernatant. A similar problem was reported by Wayne and Diaz (31) in their study on immunological distances of mycobacterial catalases. To overcome this problem, and also with an aim to improve the sensitivity, stability of reagents used in the assay, and to handle more samples at the same time without difficulty, wc have developed in this study an ELISA technique to measure the immunological crossreactivity using purified SOD enzyme as antigen. It should be emphasized that purity of the SOD will be important for such an analysis. The purification technique described in this study appears to be appropriate for this purpose, as evidenced by the final single band of proteins staining (PAGE and SDS-PAGE) and matching achromatic bands of SOD activity on PAGE (28). Also, Ouchterlony's double diffusion method showed only a single precipitation line when the antibodies were allowed to react with the purified SOD enzyme or with crude extracts of different mycobacteria. Further, there was no precipitation line between antibodies against mycobacterial SOD and SODs from Escherichia coli and armadillo liver (28). This is also substantiated by observing similar trends of relatedness by the inimunoprecipitation method based on measurement of the enzyme activity. However, it may also be of interest to check the purity of these antigen preparations by other techniques, such as Western blot, or any other analytical method.
In this ELISA method, the protein concentration (25 µ g/ml) of purified enzyme (SOD) was standardized and used as a constant for all mycobacterial SOD versus anti-SOD antibody titrations. The titers obtained by ELISA in the present study were higher than those obtained by the inimunoprecipitation technique. The high serological titers by this method may be because of the stability of the SOD as antigen rather than as enzyme and, also because of the inherent sensitivity ofthe ELISA technique. Even though high titers were obtained with this ELISA, the ImD estimation between any two different mycobacterial SODs were found to be very similar and comparable by both the immunoprecipitation and ELISA techniques. The ImDs calculated between any two mycobacterial SODs titrating either SOD enzyme versus anti-SOD antibodies or vice versa had reciprocally similar values and were reproducible. Although indications of qualitative immunological differences in SOD of Acholeplasmataceae (20) and some mycobacterial species (17-19) were suggested, the present study, for the first time, provides an ELISA for the quantitative measurement of such divergences on SOD molecules among mycobacteria. Since this study has shown the application of our ELISA system to tissue-derived M. leprae, this approach can be used for taxonomic identification of any new alleged in-vitro or in-vivo grown isolate. It is interesting to note that, by this analysis, M. leprae is closer to M. avium, M. bovis (BCG), M. tuberculosis; more distant from M. scrofulaceum and still more distant from rapid growers like M. smegmatis, M. vaccaeand M. phlei. Similar trends have been observed by the measurement of the evolutionary distance by rRNA also (29). Although M. leprae preparations did not have sufficient catalase for such analysis, other mycobacteria have exhibited similar trends of relatedness by determination of ImDs of their catalases (15). This correlation between SOD ImDs and 16S rRNA, as well as catalase ImDs, is important from the point of view of evolutionary changes. While no relatedness between M. leprae and armadillo SOD was observed (28), it would be of interest to determine the relatedness to various strains of armadilloderived mycobacteria. The results of this study, as well as the earlier reported findings of Wayne and Diaz (32), further strengthen the confidence in the use of serological measurements for determining the evolutionary relatedness/divergences of mycobacteria. SOD could be that alternate molecule for these investigations. ELISAs for B-galactosidases of lactobacilli have been described earlier (24). The results of our study further confirm the validity of this approach which can be used for various biological systems.
The stability of the reagents and the reproducible results obtained in this study show that the ELISA technique standardized in this study should be a promising method for wider application for taxonomical studies of mycobacteria (especially for the noncultivable/difficult-to-grow species/ strains), several members of which continue to be important pathogens of medical and veterinary importance. Although this technique may not be directly applicable to leprosy specimens with low bacillary load, this information shows the scope to further develop such methods based on the SOD molecule or any similar molecules. The technique can, however, be directly applied for taxonomic characterization of in vivo- (such as armadillo/nude mouse) grown strains of M. leprae as was successfully achieved earlier for M. lepraemurium using a catalasebascd system (14). By identification of specific epitopes, this strategy has the potential of developing techniques for the diagnosis and monitoring of the treatment of leprosy, other mycobacterial and, perhaps, other infectious diseases.
Acknowledgment. The authors are thankful to Dr. L. G. Wayne, VA Medical Center, Long Bcach, California, U.S.A., for valuable discussions on this study during his visit to this Institutc; to Mr. S. K. Bhan, Mr. N. S. Singh, Mr. Sri Ram, Mrs. A. Robi and the stalf of the Animal Experimental Laboratory for technical help, and to Mr. J. D. Kushwah for sccretarial assistance. The gift of some reagents by LEPRA, U.K. is gratefully acknowledged.
REFERENCES
1. BEAUCHAMP, C. and FRIDOVICH, I. Superoxide dismutase: improved assays and an assay applicable to acrylamidc gels. Anal. Biochem. 44(1971) 276-287.
2. COCKS, G. T. and WILSON, A. C. Immunological detection of single amino-acids substitutions in alkaline phosphatase. Science 164(1969)188-189.
3. COCKS, G. T. and WILSON, A. C. Enzyme evolution in the Enterobacteriaceae. J. Bacteriol. 110(1972)793-802.
4. DAVIS, B.J. Disc-clcctrophoresis method and application to human serum protein. Ann. N.Y. Acad. Sei. 121(1964)404-427.
5. DIAZ, G. A. and WAYNE, L. G. Isolation and characterization of catalasc produced by Mycobacterium tuberculosis. Am. Rev. Respir. Dis. 110(1974)312-319.
6. DRAPER, P. Protocol 1/79, Purification of M. leprae. Report on the Enlarged SC Meeting. Annex 1. Geneva: World Health Organization, 1980. TDR/IMMLER-SWG (%)/80.3.
7. GASSER, F. and GASSER, C. Immunological relationships among lactic-acid dehydrogenases in the genera Lactobacillus and Leuconosloc. J. Bacteriol. 106.(1971)113-125.
8. HOLBROOK, I. B. and LEAVER, A. G. A procedure to increase the sensitivity of staining by Coomassie brilliant blue G-250 perchloric acid solution. Anal. Biochem. 75(1976)634-636.
9. HONTEBEYRIE, M . and SSER, F. Comparative immunological relationships of two distinct sets of isofunctional dehydrogenases in the genus Leuconosloc. Int. J. Syst. Bacteriol. 25(1975)1-6.
10. HUGHES, D . E. The disintegration of microorganisms. In: Methods in Microbiology. Vol. 5B. Norris, J. R. and Ribbons, D. W., eds. London: Academic Press, 1961, pp. 1-54.
11. ICHIHARA, K., KUSONOSE, E., KUSONOSE, M. and MORI, T. Superoxide dismutase from Mycobacterium lepraemurium: its genome size, base ratio and homology with those of other mycobacteria. J. Bacteriol. 81(1977)1427-1433.
12. KAKKER, P., DAS, B. and VISHWANATHAN, P. N. A modified spectrophotomctric assay of superoxide dismutase. Indian J. Biochem. Biophys. 21(1884)130-134.
13. KATOCH, V. M., SHIVANNAVAR, C. T., DATTA, A. K. and SHARMA, V. D. Immunological relatedness of superoxide dismutase of Mycobactera: a new parameter for taxonomic identification and classification. In: Proceedings of Indo-U.K. Symposium. April 8-10, 1986. Katoch, V. M., ed. Agra: Coronation Press, 1987, pp. 219-226.
14. KATOCH, V. M., WAYNE, L. G. and DIAZ, G. A. Characterization of catalasc by micro-immunoprecipitation in tissue derived cells of Mycobacterium lepraemurium TMC 1701. Int. J. Syst. Bacteriol. 32(1982a)416-418.
15. KATOCH, V. M., WAYNE, L. G. and DIAZ, G. A. Serological approaches for the characterization of catalase in tissue derived Mycobacterium leprae. Ann. Microbiol. (Inst. Pasteur) 133B (1982b)407-414.
16. KEELE, B. B., JR., MCCORD, J. M. and FRIDOVICH, I. Superoxide dismutasc from E. coli B: a new manganese containing enzymes. J. Biol. Chem. 245(1970)617-618.
17. KUSONOSE, E., ICHIHARA, K., NADA, Y. and KUSONOSE, M. Superoxidedismutase from M. tuberculosis. J. Bactcriol. 80(1976) 1342-1352.
18. KUSONOSE, E., KUSONOSE, M., ICHIHARA, K. and IZUMI, S. Superoxide dismutase in cell free extracts from M. leprae grown in armadillo liver. FEMS Microbiol. Lett. 10(1981)9-52.
19. KUSUNOSE, M., NODA, Y., ICHIHARA, K. and KUSUNOSE, E. Superoxide dismutase from Mycobacterium species, strain Takeo. Arch. Microbiol. 108(1976)65-73.
20. LEE, G. Y. and KENNY, G. E. Immunological heterogeneity of superoxide dismutases in the Acholeplasmataccac. Int. J. Syst. Bactcriol. 34(1984)74-76.
21. LONDON, J., MEYER, E. Y. and KULKZYK, S. Comparative biochemical and immunological study of malic enzyme from two species of lactic acid bacteria: evolutionary implications. J. Bactcriol. 106(1971)126-137.
22. LOWENSTEIN, E. Die mcthodik dcr reinkulture von tuberkelbacilles aus dem Blute. Dtsch. Med. Wochenschr. 56(1930)1010.
23. MAYER, B. K. and FALKINHAM, J. O., III. Superoxide dismutase activity of Mycobacterium avium. M. intracellulare and M. scrofulaccum. Infect. Immun. 53(1986)631-635.
24. NADER DE MACIAS, M. E., PERDION, G., OLIVER, G. and HOLGADO, A. E. Enzyme linked immunosorbent assay (ELISA) for determining immunological relationships among beta-galactosidases from Lactobacilli. System. Appl. Microbiol. 8(1986)28-31.
25. NISHIKIMI, M., RAO, N. A . and YOGI, K. The occurrence of superoxide ion in the reduction of reduced phenazine methosulphate and molecular oxygen. Biochem. Biophys. Res. Commun. 46(1972)849-854.
26. PRAGER, E. M. and WILSON, A. C. The dependence of immunological cross reactivity upon sequence resemblance among lysozymes. J. Biol. Chem. 246(1971)5978-5989.
27. SAUTON, B. Sur la nutrition mincralc du bacille tubcrculeux. CR Seances Acad. Sci. 155(1912)860.
28. SHIVANNAVAR, C. T. Studies on the immunological relatedness of superoxide dismutases of mycobacteria. Ph .D. thesis, Agra University, Agra, India, 1993.
29. SMIDA, J., KAZDA, J. and STACKEBRANDT, E. Molecular genetic evidence for the relationship of Mycobacterium leprae to slow-growing pathogenic mycobacteria. Int. J. Lepr. 56(1988)449-454.
30. STANIER, R. Y., WACHTER, D., GASSER, C. and WILSON, A. C. Comparative immunological studies of two I'seudomonas enzymes. J. Bactcriol. 102(1970)351-362.
31. WAYNE, L. G. and DIAZ, G. A. Immunoprecipitation studies of mycobacterial catalase. Int. J. Syst. Bactcriol. 26(1976)38-44.
32. WAYNE, L. G. and DIAZ, G. A. Reciprocal immunological distances of catalase derived from strains of Mycobacterium avium. Mycobacterium tuberculosis and closely related species. Int. J. Syst. Bactcriol. 29(1979)19-34.
33. WHEELER. P. R. and GREGORY, D. Superoxide dismutase, pcroxidatic activity, and catalase in Mycobacterium leprae purified from armadillo liver. J. Gen. Microbiol. 121(1980)457-464.
34. Voller, A., Bidwell, D. and BARLETT, A. Enzymelinked immunosorbent assay. In: Manual of Clinical Immunology. 2ndedn. Rose, N. R. and Frucdman. A., eds. Washington, D.C. : American Society for Microbiology, 1980, pp. 359-371.
1. Ph.D., Research Assistant; Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
2. M.D., Deputy Director; Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
3. Ph.D., Research Officer; Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
4. Ph.D., former Research Assistant; Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
5. M.D., Assistant Director; Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
6. Ph.D., Officer-in-Charge (retired), Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
7. M.D., Professor and Head, Department of Microbiology, S. N. Medical College, Agra 282001, India.
Reprint requests to Dr. V. M. Katoch.
Received for publication on 23 May 1995;
Accepted for publication in revised form on 8 November 1995.